European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.ejmech.2018.01.009 Ahmed M. Gouda , Hoda A. El-Ghamry , Tahani M. Bawazeer , Thoraya A. Farghaly , Ashraf N. Abdalla , Akhmed Aslam
|
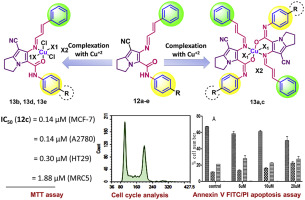
Two novel series including Schiff bases of the pyrrolizine-5-carboxamides and their Cu(II) complexes were designed, synthesized and analysed using spectral and analytical techniques. The analytical results indicated the formation of the complexes in 1:1 or 1:2 (Metal:Ligand) ratio. The geometry around the Cu centers was confirmed to be tetrahedral or octahedral. The cytotoxic activity of the new compounds was evaluated using MCF-7 (human breast adenocarcinoma), A2780 (human ovary adenocarcinoma) and HT29 (human colon adenocarcinoma), in addition to MRC5 (normal human fetal lung fibroblast) cells using the MTT cytotoxicity assay. The Schiff base 12c and the Cu complex 13b were the most active in the two series with IC50 values in the range of 0.14–2.54 μM against the three cell lines. Also, the Cu complex 13e showed excellent activity against HT29 with IC50 = 0.05μM. 7-Cyano-N-(4-methoxyphenyl)-6-((3-phenylallylidene) amino)-2,3-dihydro-1H-pyrrolizine-5-carboxamide (12c) showed high selectivity (6–13 folds) for cancerous cells over normal cells; and it induced marginal increases in the G1 and S phases of MCF-7 cells during cell cycle analysis, while compound 13b increased the MCF-7 Sub-G1 proapoptotic population, and blocked cells in the G2-M phase in a dose dependent manner. The annexin V apoptosis assay revealed the ability of compounds 12c and 13b to increase the early apoptotic MCF-7 cell populations two and three fold, respectively. Furthermore, these findings were supported by data showing that the two compounds (12c and 13b) elicit cytotoxic activity. Taken together, the data presented in this study warrants further in vitro and in vivo investigations.
中文翻译:

吡咯利嗪及其铜(II)配合物的抗肿瘤活性:具有潜在的细胞凋亡诱导活性的设计,合成和细胞毒性筛选。
使用光谱和分析技术设计,合成和分析了两个新颖的系列,包括吡咯嗪5-甲酰胺的席夫碱及其Cu(II)配合物。分析结果表明以1:1或1:2(金属:配体)的比例形成了配合物。Cu中心周围的几何形状被确认为四面体或八面体。除了MRC5(正常人胎儿肺成纤维细胞)细胞外,还使用MCF-7(人乳腺腺癌),A2780(人卵巢腺癌)和HT29(人结肠腺癌)对新化合物的细胞毒活性进行了MTT细胞毒性试验。 。Schiff碱12c和Cu络合物13b在具有IC 50的两个系列中最活跃相对于三种细胞系,其值在0.14–2.54μM的范围内。另外,Cu配合物13e对HT29表现出优异的活性,IC 50 =0.05μM。7-氰基-N-(4-甲氧基苯基)-6-((3-苯基亚芳基)氨基)-2,3-二氢-1 H-吡咯烷嗪-5-羧酰胺(12c)对苯丙胺具有很高的选择性(6-13倍)癌细胞超过正常细胞;并且它在细胞周期分析期间诱导了MCF-7细胞的G1期和S期的边缘增加,而化合物13b以剂量依赖的方式增加了MCF-7 Sub-G1细胞的凋亡,并阻断了G2-M期的细胞。Annexin V凋亡测定法揭示了化合物12c和13b的能力使早期凋亡的MCF-7细胞群体分别增加2倍和3倍。此外,这些发现得到了数据的支持,该数据表明这两种化合物(12c和13b)具有细胞毒性活性。综上所述,本研究中提出的数据值得进一步的体外和体内研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号