当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to the enantiopure pyrrolobenzodiazepine (PBD) dilactam nucleus via self-disproportionation of enantiomers
Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-08 , DOI: 10.1016/j.tet.2018.01.006 Sònia Abás , Carlos Arróniz , Elies Molins , Carmen Escolano
中文翻译:

通过对映异构体的自歧化作用获得对映纯的吡咯并苯并二氮杂(PBD)双内酰胺核
更新日期:2018-01-08
Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-08 , DOI: 10.1016/j.tet.2018.01.006 Sònia Abás , Carlos Arróniz , Elies Molins , Carmen Escolano
|
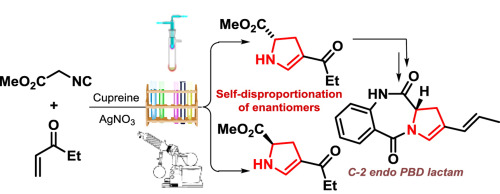
A short synthesis of an enantiopure pyrrolobenzodiazepine (PBD) dilactam featuring early installation of the C2-C3 unsaturation is reported. An enantioselective cooperative catalytic cascade followed by self-disproportionation of enantiomers via sublimation afforded the enantiopure 2,3-dihydro-1H-pyrrole key intermediate, 1. N-Acylation followed by reduction and lactam formation furnished the PBD dilactam.
中文翻译:

通过对映异构体的自歧化作用获得对映纯的吡咯并苯并二氮杂(PBD)双内酰胺核
据报道,对映体纯的吡咯并苯并二氮杂(PBD)地内酰胺的合成很短,其特征是早期安装了C2-C3不饱和键。对映选择性协同催化级联反应,然后通过升华使对映异构体自歧化,得到对映纯2,3-二氢-1 H-吡咯关键中间体,1。N-酰化,然后还原和形成内酰胺,提供了PBD地内酰胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号