当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Analysis of the Quaternary Structure of Hemoglobin Beckman Variant and Molecular Interpretation of Its Functional Abnormality: A Mass‐Spectrometry‐Based Approach
ChemBioChem ( IF 3.2 ) Pub Date : 2018-02-09 , DOI: 10.1002/cbic.201700491 Monita Muralidharan 1 , Rajdeep Das 1 , Vijay Bhat 2 , Amit Kumar Mandal 1
ChemBioChem ( IF 3.2 ) Pub Date : 2018-02-09 , DOI: 10.1002/cbic.201700491 Monita Muralidharan 1 , Rajdeep Das 1 , Vijay Bhat 2 , Amit Kumar Mandal 1
Affiliation
|
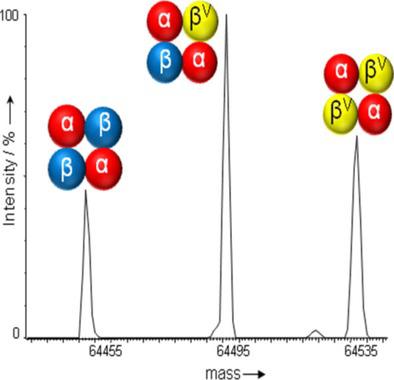
Electrostatic attraction holds the tetrameric human hemoglobin (α2β2) subunits together. The additional negative charge on hemoglobin (Hb) Beckman (βA135D) allows βV to bind the α globin chain more tightly than normal β. Although local perturbations were evident from H/DX kinetics, the quaternary structure of the hemoglobin tetramer, measured through collision cross‐section, was unaltered.

中文翻译:

血红蛋白贝克曼变体的四级结构分析及其功能异常的分子解释:基于质谱的方法
静电吸引保持所述四聚体的人血红蛋白(α 2 β 2)亚基在一起。上血红蛋白(Hb)贝克曼(βA135D)的额外的负电荷使β V到α球蛋白链比正常更β牢固地结合。尽管从H / DX动力学可以明显看出局部扰动,但通过碰撞截面测量的血红蛋白四聚体的四级结构没有改变。

更新日期:2018-02-09

中文翻译:

血红蛋白贝克曼变体的四级结构分析及其功能异常的分子解释:基于质谱的方法
静电吸引保持所述四聚体的人血红蛋白(α 2 β 2)亚基在一起。上血红蛋白(Hb)贝克曼(βA135D)的额外的负电荷使β V到α球蛋白链比正常更β牢固地结合。尽管从H / DX动力学可以明显看出局部扰动,但通过碰撞截面测量的血红蛋白四聚体的四级结构没有改变。




























 京公网安备 11010802027423号
京公网安备 11010802027423号