当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of N-Substituted 3-Amino-4-halopyridines: A Sequential Boc-Removal/Reductive Amination Mediated by Brønsted and Lewis Acids
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.joc.7b02966 Christopher A. Wilhelmsen 1 , Alexandre D.C. Dixon 1 , John D. Chisholm 1 , Daniel A. Clark 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.joc.7b02966 Christopher A. Wilhelmsen 1 , Alexandre D.C. Dixon 1 , John D. Chisholm 1 , Daniel A. Clark 1
Affiliation
|
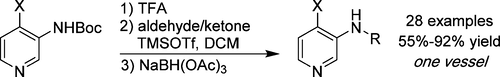
N-Substituted 3-amino-4-halopyridines are valuable synthetic intermediates, as they readily provide access to imidazopyridines and similar heterocyclic systems. The direct synthesis of N-substituted 3-amino-4-halopyridines is problematic, as reductive aminations and base-promoted alkylations are difficult in these systems. A high yielding deprotection/alkylation protocol mediated by trifluoroacetic acid and trimethylsilyl trifluoromethanesulfonate is described, providing access to a wide scope of N-substituted 3-amino-4-halopyridines. This protocol furnishes many reaction products in high purity without chromatography. Similar reductive amination conditions were also established for deactivated anilines.
中文翻译:

N取代的3-氨基-4-卤代吡啶的合成:由Brønsted和Lewis酸介导的顺序Boc去除/还原胺化
N-取代的3-氨基-4-卤代吡啶是有价值的合成中间体,因为它们易于提供与咪唑并吡啶和类似杂环系统的接触。N-取代的3-氨基-4-卤代吡啶的直接合成是有问题的,因为在这些体系中还原性胺化和碱促进的烷基化是困难的。描述了由三氟乙酸和三甲基甲硅烷基三氟甲磺酸酯介导的高产率的脱保护/烷基化方案,其提供了广泛范围的N-取代的3-氨基-4-卤代吡啶。该方案无需色谱即可提供许多高纯度的反应产物。还为失活的苯胺建立了类似的还原胺化条件。
更新日期:2018-01-19
中文翻译:

N取代的3-氨基-4-卤代吡啶的合成:由Brønsted和Lewis酸介导的顺序Boc去除/还原胺化
N-取代的3-氨基-4-卤代吡啶是有价值的合成中间体,因为它们易于提供与咪唑并吡啶和类似杂环系统的接触。N-取代的3-氨基-4-卤代吡啶的直接合成是有问题的,因为在这些体系中还原性胺化和碱促进的烷基化是困难的。描述了由三氟乙酸和三甲基甲硅烷基三氟甲磺酸酯介导的高产率的脱保护/烷基化方案,其提供了广泛范围的N-取代的3-氨基-4-卤代吡啶。该方案无需色谱即可提供许多高纯度的反应产物。还为失活的苯胺建立了类似的还原胺化条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号