当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N,O-Ligated Cu6 Cage Phenylsilsesquioxane
Organometallics ( IF 2.5 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.organomet.7b00845 Alexey N. Bilyachenko 1, 2 , Mikhail M. Levitsky 1 , Victor N. Khrustalev 2 , Yan V. Zubavichus 3 , Lidia S. Shul’pina 1 , Elena S. Shubina 1 , Georgiy B. Shul’pin 4, 5
Organometallics ( IF 2.5 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.organomet.7b00845 Alexey N. Bilyachenko 1, 2 , Mikhail M. Levitsky 1 , Victor N. Khrustalev 2 , Yan V. Zubavichus 3 , Lidia S. Shul’pina 1 , Elena S. Shubina 1 , Georgiy B. Shul’pin 4, 5
Affiliation
|
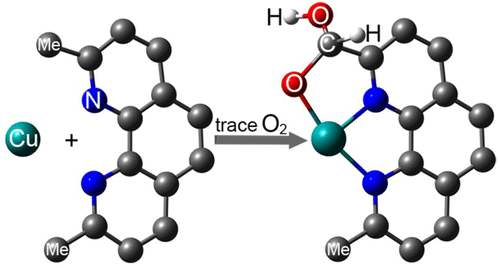
The self-assembly synthesis of Cu(II)-silsesquioxane involving 2,9-dimethyl-1,10-phenanthroline (neocuproine) as an additional N ligand at copper atoms was performed. The reaction revealed an unprecedented aerobic hydroxylation of only one of the two methyl groups in neocuproine to afford the corresponding geminal diol. The produced derivative of oxidized neocuproine acts as a two-centered N,O ligand in the assembly of the hexacopper cage product [Cu6(Ph5Si5O10)2·(C14H11N2O2)2] (1), coordinating two of the six copper centers in the product. Two siloxanolate ligands [PhSi(O)O]5 in the cis configuration coordinate to the rest of the copper(II) ions. Compound 1 is a highly efficient homogeneous precatalyst in the oxidation of alkanes and alcohols with peroxides.
中文翻译:

轻度和区域选择性的甲基新甲基异丙基羟甲基化:N,O限制Cu 6笼式苯基倍半硅氧烷的方法
进行了Cu(II)-倍半硅氧烷的自组装合成,其中涉及2,9-二甲基-1,10-菲咯啉(新古嘌呤)作为在铜原子上的附加N配体。该反应表明新cuproine中仅两个甲基之一发生了前所未有的好氧羟基化反应,从而生成了相应的双生二醇。在六铜笼式产物[Cu 6(Ph 5 Si 5 O 10)2 ·(C 14 H 11 N 2 O 2)2 ](1个),协调产品中六个铜中心中的两个。两个顺式构型的硅氧烷醇盐配体[PhSi(O)O] 5与其余的铜(II)离子配位。在用过氧化物氧化烷烃和醇中,化合物1是高效的均相预催化剂。
更新日期:2018-01-08
中文翻译:

轻度和区域选择性的甲基新甲基异丙基羟甲基化:N,O限制Cu 6笼式苯基倍半硅氧烷的方法
进行了Cu(II)-倍半硅氧烷的自组装合成,其中涉及2,9-二甲基-1,10-菲咯啉(新古嘌呤)作为在铜原子上的附加N配体。该反应表明新cuproine中仅两个甲基之一发生了前所未有的好氧羟基化反应,从而生成了相应的双生二醇。在六铜笼式产物[Cu 6(Ph 5 Si 5 O 10)2 ·(C 14 H 11 N 2 O 2)2 ](1个),协调产品中六个铜中心中的两个。两个顺式构型的硅氧烷醇盐配体[PhSi(O)O] 5与其余的铜(II)离子配位。在用过氧化物氧化烷烃和醇中,化合物1是高效的均相预催化剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号