当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ambiphilic Molecules: From Organometallic Curiosity to Metal-Free Catalysts
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.accounts.7b00514 Frédéric-Georges Fontaine 1 , Étienne Rochette 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.accounts.7b00514 Frédéric-Georges Fontaine 1 , Étienne Rochette 1
Affiliation
|
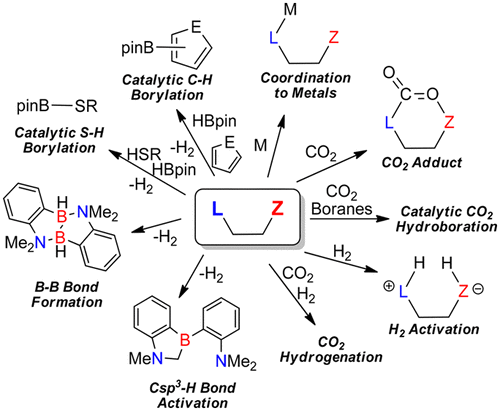
Ambiphilic molecules were first used as functional ligands for transition elements, which could enable intriguing organometallic transformations. In the past decade, these intramolecular Lewis pairs, first considered organometallic curiosities, have become staples in organometallic chemistry and catalysis, acting as Z ligands, activating inert molecules using the concept of frustrated Lewis pair (FLP) chemistry, and acting as metal-free catalysts. In this Account, we detail our contribution to this blossoming field of research, focusing on the use of ambiphilic molecules as metal-free catalysts for CO2 reduction and C–H borylation reactions. A major emphasis is put on the mechanistic investigations we carried out using reactivity studies and theoretical tools, which helped us steer our research from stoichiometric transformations to highly active catalytic processes. We first report the interaction of aluminum-phosphine ambiphilic molecules with carbon dioxide. Although these Lewis pairs can bind CO2, a study of the deactivation process in the presence of CO2 and hydroboranes led us to discover that simple phosphinoborane molecules could act as active precatalysts for the hydroboration of carbon dioxide into methanol precursors. In these systems, the Lewis basic sites interact with the reducing agents rather than with the electrophilic carbon of CO2, increasing the nucleophilicity of hydroboranes. Simultaneously, the weak Lewis acids stabilize the oxygen of the gas molecule in the transition state, leading to high reaction rates. Replacing the phosphine by an amine leads to a system enabling CO2 hydrogenation, albeit only in stoichiometric transformations. Investigation of the protodeborylation deactivation of aminoboranes led us to develop metal-free catalysts for the C–H borylation of heteroarenes. By protecting the Lewis acid sites of these catalysts using fluoride, we were able to synthesize practical, air-stable precatalysts allowing the convenient synthesis of heteroarylboronic esters on a multigram scale. Contrary to general perception of FLP chemistry, we also demonstrated that a significant increase in activity could be obtained by reducing the steric bulk around the active site. These smaller systems exist as stable dimers and are more energetically costly to dissociate into active FLPs, but the approach of the substrate and the C–H activation step are significantly favored compared to the bulkier analogues. An in-depth study of the stability and reactivity of these aminoborane molecules also allowed us to develop a metal-free catalytic S–H bond borylation system, and to report stoichiometric and spontaneous B–B bond formation and Csp3-H bond activation processes, highlighting the importance of H2 release as a thermodynamic driving force in these FLP transformations.
中文翻译:

两性分子:从有机金属好奇心到无金属催化剂
两性分子首先被用作过渡元素的功能性配体,这可以使有机金属转化变得有趣。在过去的十年中,这些首先被认为是有机金属好奇心的分子内路易斯对,已成为有机金属化学和催化的重要组成部分,充当Z配体,使用沮丧的路易斯对(FLP)化学概念激活惰性分子,并且不含金属催化剂。在此报告中,我们详细介绍了我们在这一蓬勃发展的研究领域中所做出的贡献,着重于使用歧义性分子作为CO 2的无金属催化剂还原和C–H硼化反应。我们重点研究了使用反应性研究和理论工具进行的机理研究,这有助于我们将研究方向从化学计量转化为高活性催化过程。我们首先报道铝膦双歧性分子与二氧化碳的相互作用。尽管这些路易斯对可以结合CO 2,但是对在CO 2和氢硼烷存在下的失活过程的研究使我们发现,简单的膦硼烷分子可以用作将二氧化碳氢硼化为甲醇前体的活性预催化剂。在这些系统中,路易斯碱性位点与还原剂相互作用,而不是与CO 2的亲电子碳相互作用,增加了氢硼烷的亲核性。同时,弱路易斯酸将气体分子的氧稳定在过渡态,从而导致高反应速率。用胺代替磷化氢导致生成CO 2的系统氢化,尽管仅是化学计量的转化。对氨基硼烷的原去去硼化反应的研究使我们开发了用于杂芳烃的CH硼化的无金属催化剂。通过使用氟化物保护这些催化剂的路易斯酸位,我们能够合成实用的,空气稳定的预催化剂,从而可以在数克规模上方便地合成杂芳基硼酸酯。与FLP化学的一般认识相反,我们还证明,通过减少活性位点周围的空间体积,可以显着提高活性。这些较小的系统以稳定的二聚体形式存在,并且解离成活性FLP的能量消耗更高,但是与体积较大的类似物相比,底物的方法和C–H活化步骤受到了极大的青睐。3- H键活化过程,突出了在这些FLP转化中H 2释放作为热力学驱动力的重要性。
更新日期:2018-01-08
中文翻译:

两性分子:从有机金属好奇心到无金属催化剂
两性分子首先被用作过渡元素的功能性配体,这可以使有机金属转化变得有趣。在过去的十年中,这些首先被认为是有机金属好奇心的分子内路易斯对,已成为有机金属化学和催化的重要组成部分,充当Z配体,使用沮丧的路易斯对(FLP)化学概念激活惰性分子,并且不含金属催化剂。在此报告中,我们详细介绍了我们在这一蓬勃发展的研究领域中所做出的贡献,着重于使用歧义性分子作为CO 2的无金属催化剂还原和C–H硼化反应。我们重点研究了使用反应性研究和理论工具进行的机理研究,这有助于我们将研究方向从化学计量转化为高活性催化过程。我们首先报道铝膦双歧性分子与二氧化碳的相互作用。尽管这些路易斯对可以结合CO 2,但是对在CO 2和氢硼烷存在下的失活过程的研究使我们发现,简单的膦硼烷分子可以用作将二氧化碳氢硼化为甲醇前体的活性预催化剂。在这些系统中,路易斯碱性位点与还原剂相互作用,而不是与CO 2的亲电子碳相互作用,增加了氢硼烷的亲核性。同时,弱路易斯酸将气体分子的氧稳定在过渡态,从而导致高反应速率。用胺代替磷化氢导致生成CO 2的系统氢化,尽管仅是化学计量的转化。对氨基硼烷的原去去硼化反应的研究使我们开发了用于杂芳烃的CH硼化的无金属催化剂。通过使用氟化物保护这些催化剂的路易斯酸位,我们能够合成实用的,空气稳定的预催化剂,从而可以在数克规模上方便地合成杂芳基硼酸酯。与FLP化学的一般认识相反,我们还证明,通过减少活性位点周围的空间体积,可以显着提高活性。这些较小的系统以稳定的二聚体形式存在,并且解离成活性FLP的能量消耗更高,但是与体积较大的类似物相比,底物的方法和C–H活化步骤受到了极大的青睐。3- H键活化过程,突出了在这些FLP转化中H 2释放作为热力学驱动力的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号