当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionothermal synthesis of magnetically-retrievable mesoporous carbons from alkyne-appended ionic liquids and demonstration of their use in selective dye removal†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7nj03849f Sudhir Ravula 1, 2, 3, 4 , Nathaniel E. Larm 1, 2, 3, 4 , Yi Liu 1, 2, 3, 4 , Jerry L. Atwood 1, 2, 3, 4 , Sheila N. Baker 1, 2, 3, 4 , Gary A. Baker 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7nj03849f Sudhir Ravula 1, 2, 3, 4 , Nathaniel E. Larm 1, 2, 3, 4 , Yi Liu 1, 2, 3, 4 , Jerry L. Atwood 1, 2, 3, 4 , Sheila N. Baker 1, 2, 3, 4 , Gary A. Baker 1, 2, 3, 4
Affiliation
|
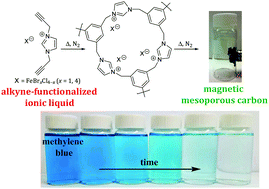
We have devised a simple, convenient, and template-free synthetic strategy to make inherently functional (magnetically-responsive) mesoporous carbons by single-step ionothermal cyclotrimerization of alkyne-functionalized ionic liquids (AFILs) comprising paramagnetic anions. During the carbonization process, bromotrichloroferrate(III) [FeBrCl3]− or tetrabromoferrate(III) [FeBr4]− anions act as catalysts in controlling the textural properties of carbon, whereas the cation acts as a carbon source. The resulting mesoporous carbons were probed using a host of tools, including X-ray diffraction, thermogravimetric analysis, nitrogen porosimetry (BET analysis), and transmission electron microscopy. Depending on the nature of the parent anion, magnetically-responsive mesoporous carbons (Fe-AFILs@C) with specific surface areas as high as 544 m2 g−1 and good structural stability were obtained from the ionothermal synthesis. The current AFILs are shown to be viable precursors to mesoporous carbons with interesting potential applications. For instance, we demonstrate the sorption of the cationic dye methylene blue (MB) and the anionic dye thiazine red R (TRR) from a contaminated aqueous stream using Fe-AFILs@C as well as their subsequent degradation via Fenton chemistry. The detailed sorption kinetics and the isotherm model for the equilibrium process were investigated and are in accordance with pseudo-second-order kinetics for a Langmuir-type adsorption isotherm. Interestingly, the mesoporous carbons were also successfully applied as selective sorbents to separate a binary mixture of the MB and TRR dyes. Significantly, the Fe-AFILs@C can be easily separated from the aqueous solution following the sorption process and can be easily regenerated using a simple ethanol washing step which releases the dye.
中文翻译:

从附炔的离子液体中电热合成磁性可回收的中孔碳,并证明其可用于选择性去除染料†
我们已经设计出一种简单,方便且无模板的合成策略,可通过包含顺磁性阴离子的炔烃官能化离子液体(AFIL)的一步电离热三聚来制备具有固有功能(磁响应)的中孔碳。在碳化过程中,溴化三氯高铁酸盐(III) [FeBrCl 3 ] -或四溴高铁酸盐(III) [FeBr 4 ] -阴离子在控制碳的织构特性方面起催化剂的作用,而阳离子则作为碳源。使用许多工具探测所得的介孔碳,这些工具包括X射线衍射,热重分析,氮孔隙率法(BET分析)和透射电子显微镜。取决于母体阴离子的性质,比表面积高达544 m 2 g -1的磁响应中孔碳(Fe-AFILs @ C)电离合成获得了良好的结构稳定性。当前的AFIL被证明是中孔碳的可行前体,具有令人感兴趣的潜在应用。例如,我们证明了使用Fe-AFILs @ C从受污染的水流中吸附阳离子染料亚甲基蓝(MB)和阴离子染料噻嗪红R(TRR)以及它们随后的降解通过Fenton化学。研究了平衡过程的详细吸附动力学和等温线模型,并与Langmuir型吸附等温线的拟二级动力学相符。有趣的是,中孔碳也成功地用作选择性吸附剂,以分离MB和TRR染料的二元混合物。重要的是,Fe-AFILs @ C在吸附过程之后可以很容易地从水溶液中分离出来,并且可以使用简单的乙醇洗涤步骤轻松地再生,从而释放出染料。
更新日期:2018-01-08
中文翻译:

从附炔的离子液体中电热合成磁性可回收的中孔碳,并证明其可用于选择性去除染料†
我们已经设计出一种简单,方便且无模板的合成策略,可通过包含顺磁性阴离子的炔烃官能化离子液体(AFIL)的一步电离热三聚来制备具有固有功能(磁响应)的中孔碳。在碳化过程中,溴化三氯高铁酸盐(III) [FeBrCl 3 ] -或四溴高铁酸盐(III) [FeBr 4 ] -阴离子在控制碳的织构特性方面起催化剂的作用,而阳离子则作为碳源。使用许多工具探测所得的介孔碳,这些工具包括X射线衍射,热重分析,氮孔隙率法(BET分析)和透射电子显微镜。取决于母体阴离子的性质,比表面积高达544 m 2 g -1的磁响应中孔碳(Fe-AFILs @ C)电离合成获得了良好的结构稳定性。当前的AFIL被证明是中孔碳的可行前体,具有令人感兴趣的潜在应用。例如,我们证明了使用Fe-AFILs @ C从受污染的水流中吸附阳离子染料亚甲基蓝(MB)和阴离子染料噻嗪红R(TRR)以及它们随后的降解通过Fenton化学。研究了平衡过程的详细吸附动力学和等温线模型,并与Langmuir型吸附等温线的拟二级动力学相符。有趣的是,中孔碳也成功地用作选择性吸附剂,以分离MB和TRR染料的二元混合物。重要的是,Fe-AFILs @ C在吸附过程之后可以很容易地从水溶液中分离出来,并且可以使用简单的乙醇洗涤步骤轻松地再生,从而释放出染料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号