当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the turn-inducing properties of asparagine: the structuring role of the amide side chain, from isolated model peptides to crystallized proteins†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cp07605c S. Habka 1, 2, 3, 4, 5 , W. Y. Sohn 1, 2, 3, 4, 5 , V. Vaquero-Vara 1, 2, 3, 4, 5 , M. Géléoc 1, 2, 3, 4, 5 , B. Tardivel 1, 2, 3, 4, 5 , V. Brenner 1, 2, 3, 4, 5 , E. Gloaguen 1, 2, 3, 4, 5 , M. Mons 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cp07605c S. Habka 1, 2, 3, 4, 5 , W. Y. Sohn 1, 2, 3, 4, 5 , V. Vaquero-Vara 1, 2, 3, 4, 5 , M. Géléoc 1, 2, 3, 4, 5 , B. Tardivel 1, 2, 3, 4, 5 , V. Brenner 1, 2, 3, 4, 5 , E. Gloaguen 1, 2, 3, 4, 5 , M. Mons 1, 2, 3, 4, 5
Affiliation
|
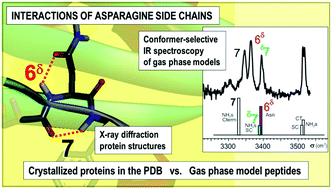
Asparagine (Asn) is a powerful turn-inducer residue, with a large propensity to occupy the second position in the central region of β-turns of proteins. The present work aims at investigating the role of a local anchoring between the Asn side chain and the main chain in this remarkable property. For this purpose, the H-bonding patterns of an asparagine residue in an isolated protein chain fragment forming a γ- or a β-turn have been determined using IR/UV double resonance gas phase spectroscopy on laser-desorbed, jet-cooled short models in conjunction with relevant quantum chemistry calculations. These gas phase data provide evidence for an original double anchoring linking the Asn primary amide side chain (SC), which adopts a gauche+ rotameric form, to its main chain (MC) local environment. From both IR spectroscopic evidence (H-bond induced red shifts) and quantum chemistry, Asn SC is found to behave as a stronger H-bond acceptor than donor, resulting in stronger MC→SC H-bonds than SC→MC ones. These gas phase structural data, relevant to a hydrophobic environment, have been used as a reference to assess the anchoring taking place in high resolution crystallized proteins of the Protein Data Bank. This approach reveals that, when the SC adopts a gauche+ orientation, the stronger MC→SC bonds are preserved in many cases whereas the SC→MC bonds are always disrupted, in qualitative agreement with the gas phase ranking of these interactions. Most interestingly, when Asn occupies the second position of central part of a β-turn (i.e., the very turn-inducer position), the MC→SC H-bonds are also disrupted and replaced by a water-mediated SC to MC anchoring. Owing to the specific features of the hydrated Asn side chain, we propose that it could be a turn precursor structure, able to facilitate turn formation in the early events of the folding process.
中文翻译:

关于天冬酰胺的诱导特性:酰胺侧链的结构作用,从分离的模型肽到结晶蛋白†
天冬酰胺(Asn)是强大的诱导剂残基,具有很大的倾向占据蛋白质β-turns中心区域的第二个位置。目前的工作旨在调查在这种非同寻常的特性中,Asn侧链和主链之间的局部锚固的作用。为此,已使用IR / UV双共振气相色谱法在激光解吸的射流冷却短模型上确定了分离的蛋白链片段中形成γ或β角的天冬酰胺残基的H键模式。结合相关的量子化学计算。这些气相数据为连接Asn伯酰胺侧链(SC)的原始双锚定提供了证据,后者采用纱布+旋转异构形式,以其主链(MC)本地环境为准。从红外光谱证据(氢键引起的红移)和量子化学的角度来看,Asn SC被认为是比供体更强的氢键受体,从而导致MC→SC的氢键比SC→MC的强。这些与疏水环境有关的气相结构数据已被用作评估锚定在蛋白质数据库高分辨率结晶蛋白质中发生的参考。该方法表明,当SC采用薄纱+取向时,在许多情况下会保留更强的MC→SC键,而SC→MC键始终被打断,这与这些相互作用的气相定性定性一致。最有趣的是,当Asn占据β圈中心位置的第二个位置时(也就是说,MC→SC的H键也被破坏,并被水介导的SC固定在MC上。由于水合Asn侧链的特定特征,我们建议它可以是转弯前体结构,能够在折叠过程的早期事件中促进转弯形成。
更新日期:2018-01-08
中文翻译:

关于天冬酰胺的诱导特性:酰胺侧链的结构作用,从分离的模型肽到结晶蛋白†
天冬酰胺(Asn)是强大的诱导剂残基,具有很大的倾向占据蛋白质β-turns中心区域的第二个位置。目前的工作旨在调查在这种非同寻常的特性中,Asn侧链和主链之间的局部锚固的作用。为此,已使用IR / UV双共振气相色谱法在激光解吸的射流冷却短模型上确定了分离的蛋白链片段中形成γ或β角的天冬酰胺残基的H键模式。结合相关的量子化学计算。这些气相数据为连接Asn伯酰胺侧链(SC)的原始双锚定提供了证据,后者采用纱布+旋转异构形式,以其主链(MC)本地环境为准。从红外光谱证据(氢键引起的红移)和量子化学的角度来看,Asn SC被认为是比供体更强的氢键受体,从而导致MC→SC的氢键比SC→MC的强。这些与疏水环境有关的气相结构数据已被用作评估锚定在蛋白质数据库高分辨率结晶蛋白质中发生的参考。该方法表明,当SC采用薄纱+取向时,在许多情况下会保留更强的MC→SC键,而SC→MC键始终被打断,这与这些相互作用的气相定性定性一致。最有趣的是,当Asn占据β圈中心位置的第二个位置时(也就是说,MC→SC的H键也被破坏,并被水介导的SC固定在MC上。由于水合Asn侧链的特定特征,我们建议它可以是转弯前体结构,能够在折叠过程的早期事件中促进转弯形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号