当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nine-step total synthesis of (−)-strychnofoline
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cc08938d Qingzhen Yu 1, 2, 3, 4, 5 , Pan Guo 1, 2, 3, 4, 5 , Jie Jian 1, 2, 3, 4, 5 , Yuye Chen 1, 2, 3, 4, 5 , Jing Xu 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cc08938d Qingzhen Yu 1, 2, 3, 4, 5 , Pan Guo 1, 2, 3, 4, 5 , Jie Jian 1, 2, 3, 4, 5 , Yuye Chen 1, 2, 3, 4, 5 , Jing Xu 1, 2, 3, 4, 5
Affiliation
|
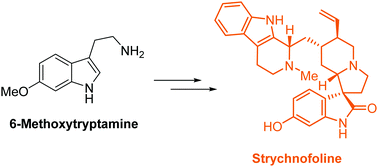
Strychnofoline is a Strychnos alkaloid that has unique spirooxindole architecture and possesses important anticancer activity. Here, we have, for the first time, reported the enantioselective synthesis of strychnofoline proceeding in only nine steps from commercially available 6-methoxytryptamine. The efficiency of the synthesis derives from the use of two sequential transformation steps in the catalytic asymmetric construction of the spiro[pyrrolidine-3,3′-oxindole] motif in a facile manner. Our route is amenable to the synthesis of other natural and synthetic analogs of bioactive spirooxindole alkaloids to access their therapeutic potential.
中文翻译:

(-)-苦瓜碱的九步全合成
Strychnofoline是一种Strychnos生物碱,具有独特的螺氧杂吲哚结构,并具有重要的抗癌活性。在这里,我们首次报道了从市售的6-甲氧基色胺仅需九步就可以合成对苯丙氨酚的对映体选择性合成。合成的效率源自在螺环[吡咯烷-3,3'-羟吲哚]基序的催化不对称结构中以简便的方式使用两个连续的转化步骤。我们的路线适合合成具有生物活性的螺氧并吲哚生物碱的其他天然和合成类似物,以发挥其治疗潜力。
更新日期:2018-01-25
中文翻译:

(-)-苦瓜碱的九步全合成
Strychnofoline是一种Strychnos生物碱,具有独特的螺氧杂吲哚结构,并具有重要的抗癌活性。在这里,我们首次报道了从市售的6-甲氧基色胺仅需九步就可以合成对苯丙氨酚的对映体选择性合成。合成的效率源自在螺环[吡咯烷-3,3'-羟吲哚]基序的催化不对称结构中以简便的方式使用两个连续的转化步骤。我们的路线适合合成具有生物活性的螺氧并吲哚生物碱的其他天然和合成类似物,以发挥其治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号