当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NHC and nucleophile chelation effects on reactive iron(ii) species in alkyl–alkyl cross-coupling†
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7sc04750a Valerie E Fleischauer 1 , Salvador B Muñoz Iii 1 , Peter G N Neate 1 , William W Brennessel 1 , Michael L Neidig 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7sc04750a Valerie E Fleischauer 1 , Salvador B Muñoz Iii 1 , Peter G N Neate 1 , William W Brennessel 1 , Michael L Neidig 1
Affiliation
|
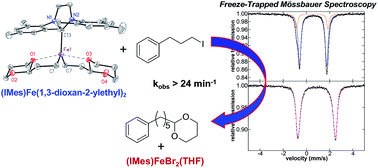
While iron–NHC catalysed cross-couplings have been shown to be effective for a wide variety of reactions (e.g. aryl–aryl, aryl–alkyl, alkyl–alkyl), the nature of the in situ formed and reactive iron species in effective catalytic systems remains largely undefined. In the current study, freeze-trapped Mössbauer spectroscopy, and EPR studies combined with inorganic synthesis and reaction studies are utilised to define the key in situ formed and reactive iron–NHC species in the Kumada alkyl–alkyl cross-coupling of (2-(1,3-dioxan-2-yl)ethyl)magnesium bromide and 1-iodo-3-phenylpropane. The key reactive iron species formed in situ is identified as (IMes)Fe((1,3-dioxan-2-yl)ethyl)2, whereas the S = 1/2 iron species previously identified in this chemistry is found to be only a very minor off-cycle species (<0.5% of all iron). Reaction and kinetic studies demonstrate that (IMes)Fe((1,3-dioxan-2-yl)ethyl)2 is highly reactive towards the electrophile resulting in two turnovers with respect to iron (kobs > 24 min−1) to generate cross-coupled product with overall selectivity analogous to catalysis. The high resistance of this catalytic system to β-hydride elimination of the alkyl nucleophile is attributed to its chelation to iron through ligation of carbon and one oxygen of the acetal moiety of the nucleophile. In fact, alternative NHC ligands such as SIPr are less effective in catalysis due to their increased steric bulk inhibiting the ability of the alkyl ligands to chelate. Overall, this study identifies a novel alkyl chelation method to achieve effective alkyl–alkyl cross-coupling with iron(II)–NHCs, provides direct structural insight into NHC effects on catalytic performance and extends the importance of iron(II) reactive species in iron-catalysed cross-coupling.
中文翻译:

NHC 和亲核试剂螯合对烷基-烷基交叉偶联中活性铁 (ii) 物种的影响†
虽然铁-NHC催化的交叉偶联已被证明对多种反应有效(例如芳基-芳基、芳基-烷基、烷基-烷基),但有效催化体系中原位形成的活性铁物种的性质很大程度上仍然是未定义的。在当前的研究中,冷冻捕集穆斯堡尔光谱和 EPR 研究与无机合成和反应研究相结合,用于定义 (2-( 1,3-二恶烷-2-基)乙基)溴化镁和1-碘-3-苯基丙烷。原位形成的关键活性铁物种被确定为 (IMes)Fe((1,3-dioxan-2-yl)ethy) 2 ,而之前在此化学中确定的S = 1/2 铁物种仅是非常小的非循环物种(<0 id=85> 2对亲电子试剂具有高度反应性,导致相对于铁的两次周转( k obs > 24 min -1 ),以生成具有类似于催化的总体选择性的交叉偶联产物该催化系统对烷基亲核试剂的β-氢化物消除的高抵抗力归因于其通过亲核试剂的缩醛部分的碳和一个氧的连接而与铁螯合。由于其增加的空间体积抑制烷基配体螯合的能力,因此具有有效的催化作用。 总体而言,本研究确定了一种新型烷基螯合方法,可实现与铁( II )–NHCs的有效烷基-烷基交叉偶联,提供了NHC对催化性能影响的直接结构见解,并扩展了铁( II )反应物种在铁中的重要性。 -催化交叉偶联。
更新日期:2018-01-08
中文翻译:

NHC 和亲核试剂螯合对烷基-烷基交叉偶联中活性铁 (ii) 物种的影响†
虽然铁-NHC催化的交叉偶联已被证明对多种反应有效(例如芳基-芳基、芳基-烷基、烷基-烷基),但有效催化体系中原位形成的活性铁物种的性质很大程度上仍然是未定义的。在当前的研究中,冷冻捕集穆斯堡尔光谱和 EPR 研究与无机合成和反应研究相结合,用于定义 (2-( 1,3-二恶烷-2-基)乙基)溴化镁和1-碘-3-苯基丙烷。原位形成的关键活性铁物种被确定为 (IMes)Fe((1,3-dioxan-2-yl)ethy) 2 ,而之前在此化学中确定的S = 1/2 铁物种仅是非常小的非循环物种(<0 id=85> 2对亲电子试剂具有高度反应性,导致相对于铁的两次周转( k obs > 24 min -1 ),以生成具有类似于催化的总体选择性的交叉偶联产物该催化系统对烷基亲核试剂的β-氢化物消除的高抵抗力归因于其通过亲核试剂的缩醛部分的碳和一个氧的连接而与铁螯合。由于其增加的空间体积抑制烷基配体螯合的能力,因此具有有效的催化作用。 总体而言,本研究确定了一种新型烷基螯合方法,可实现与铁( II )–NHCs的有效烷基-烷基交叉偶联,提供了NHC对催化性能影响的直接结构见解,并扩展了铁( II )反应物种在铁中的重要性。 -催化交叉偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号