Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Serological Point-of-Care Test for the Detection of IgG Antibodies against Ebola Virus in Human Survivors
ACS Nano ( IF 17.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acsnano.7b07021 Polina Brangel 1 , Ariel Sobarzo 2 , Claudio Parolo 3 , Benjamin S. Miller 3 , Philip D. Howes 1 , Sigal Gelkop 2 , Julius J. Lutwama 4 , John M. Dye 5 , Rachel A. McKendry 3 , Leslie Lobel 2, 4 , Molly M. Stevens 1
ACS Nano ( IF 17.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acsnano.7b07021 Polina Brangel 1 , Ariel Sobarzo 2 , Claudio Parolo 3 , Benjamin S. Miller 3 , Philip D. Howes 1 , Sigal Gelkop 2 , Julius J. Lutwama 4 , John M. Dye 5 , Rachel A. McKendry 3 , Leslie Lobel 2, 4 , Molly M. Stevens 1
Affiliation
|
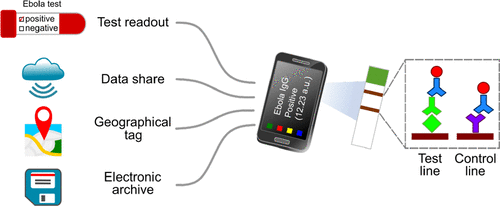
Ebola virus disease causes widespread and highly fatal epidemics in human populations. Today, there is still great need for point-of-care tests for diagnosis, patient management and surveillance, both during and post outbreaks. We present a point-of-care test comprising an immunochromatographic strip and a smartphone reader, which detects and semiquantifies Ebola-specific antibodies in human survivors. We developed a Sudan virus glycoprotein monoplex platform and validated it using sera from 90 human survivors and 31 local noninfected controls. The performance of the glycoprotein monoplex was 100% sensitivity and 98% specificity compared to standard whole antigen enzyme-linked immunosorbent assay (ELISA), and it was validated with freshly collected patient samples in Uganda. Moreover, we constructed a multiplex test for simultaneous detection of antibodies against three recombinant Sudan virus proteins. A pilot study comprising 15 survivors and 5 noninfected controls demonstrated sensitivity and specificity of 100% compared to standard ELISA. Finally, we developed a second multiplex subtype assay for the identification of exposure to three related EVD species: Sudan virus, Bundibugyo virus and Ebola virus (formerly Zaire) using recombinant viral glycoprotein. This multiplex test could distinguish between the host’s immunity to specific viral species and identify cross-reactive immunity. These developed serological platforms consisted of capture ligands with high specificity and sensitivity, in-house developed strips and a compatible smartphone application. These platforms enabled rapid and portable testing, data storage and sharing as well as geographical tagging of the tested individuals in Uganda. This platform holds great potential as a field tool for diagnosis, vaccine development, and therapeutic evaluation.
中文翻译:

血清学现场检测,用于检测人类幸存者中抗埃博拉病毒的IgG抗体
埃博拉病毒病在人类中引起广泛且高度致命的流行病。如今,在暴发期间和暴发后仍非常需要用于诊断,患者管理和监视的即时检验。我们提出了一个即时检验项目,包括一个免疫色谱条和一个智能手机阅读器,它可以检测并半定量人类幸存者中的埃博拉特异性抗体。我们开发了一个苏丹病毒糖蛋白单链平台,并使用了来自90位人类幸存者和31位局部未感染对照的血清对其进行了验证。与标准全抗原酶联免疫吸附测定(ELISA)相比,糖蛋白单链体的性能为100%灵敏度和98%特异性,并且已在乌干达进行了新采集的患者样品的验证。而且,我们构建了同时检测针对三种重组苏丹病毒蛋白的抗体的多重测试。包含15个幸存者和5个未感染对照的初步研究表明,与标准ELISA相比,其敏感性和特异性为100%。最后,我们使用重组病毒糖蛋白开发了第二种多重亚型检测方法,用于鉴定与三种相关EVD物种的接触:苏丹病毒,邦迪布约病毒和埃博拉病毒(以前的扎伊尔)。这项多重测试可以区分宿主对特定病毒物种的免疫力,并确定交叉反应性免疫力。这些发达的血清学平台包括具有高特异性和灵敏度的捕获配体,内部开发的检测条和兼容的智能手机应用程序。这些平台可实现快速,便携式的测试,数据存储和共享以及乌干达受测人员的地理标签。该平台作为诊断,疫苗开发和治疗评估的现场工具具有巨大的潜力。
更新日期:2018-01-05
中文翻译:

血清学现场检测,用于检测人类幸存者中抗埃博拉病毒的IgG抗体
埃博拉病毒病在人类中引起广泛且高度致命的流行病。如今,在暴发期间和暴发后仍非常需要用于诊断,患者管理和监视的即时检验。我们提出了一个即时检验项目,包括一个免疫色谱条和一个智能手机阅读器,它可以检测并半定量人类幸存者中的埃博拉特异性抗体。我们开发了一个苏丹病毒糖蛋白单链平台,并使用了来自90位人类幸存者和31位局部未感染对照的血清对其进行了验证。与标准全抗原酶联免疫吸附测定(ELISA)相比,糖蛋白单链体的性能为100%灵敏度和98%特异性,并且已在乌干达进行了新采集的患者样品的验证。而且,我们构建了同时检测针对三种重组苏丹病毒蛋白的抗体的多重测试。包含15个幸存者和5个未感染对照的初步研究表明,与标准ELISA相比,其敏感性和特异性为100%。最后,我们使用重组病毒糖蛋白开发了第二种多重亚型检测方法,用于鉴定与三种相关EVD物种的接触:苏丹病毒,邦迪布约病毒和埃博拉病毒(以前的扎伊尔)。这项多重测试可以区分宿主对特定病毒物种的免疫力,并确定交叉反应性免疫力。这些发达的血清学平台包括具有高特异性和灵敏度的捕获配体,内部开发的检测条和兼容的智能手机应用程序。这些平台可实现快速,便携式的测试,数据存储和共享以及乌干达受测人员的地理标签。该平台作为诊断,疫苗开发和治疗评估的现场工具具有巨大的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号