当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Soft–Hard Acid/Base-Controlled, Oxidative, N-Selective Arylation of Sulfonanilides via a Nitrenium Ion
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.joc.7b02841 Saikat Maiti 1 , Prasenjit Mal 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.joc.7b02841 Saikat Maiti 1 , Prasenjit Mal 1
Affiliation
|
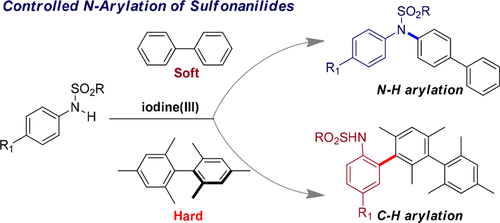
In iodine(III)-catalyzed, dehydrogenative arylations of sulfonanilides, the functionalization of C–C bonds is preferred over the functionalization of C–N bonds. Herein, an unprecedented N-selective arylation of sulfonanilides using soft–hard acid–base (SHAB) control by a nitrenium ion over a carbenium ion is reported. Treatment of sulfonanilides with iodine(III) led to the formation of nitrenium ions (soft), which preferentially react with biphenyls (soft) over bimesityl (hard) to generate C–N bonds. The iodine(III) was generated in situ by using PhI and mCPBA at room temperature.
中文翻译:

软硬酸/碱控制的,通过硝基离子对磺酰苯胺进行氧化,N选择性芳构化
在碘(III)催化的磺酰苯胺的脱氢芳基化反应中,与C–N键相比,优选C–C键的功能。在本文中,据报道,通过soft离子对over离子的软硬酸碱(SHAB)控制,对磺酰苯胺进行了空前的N选择性芳基化。用碘(III)处理磺酰苯胺会导致形成亚硝酸根离子(软),其优先于联苯(软)而不是双苯甲醚(硬)与联苯反应生成C–N键。在室温下使用PhI和m CPBA原位生成碘(III)。
更新日期:2018-01-19
中文翻译:

软硬酸/碱控制的,通过硝基离子对磺酰苯胺进行氧化,N选择性芳构化
在碘(III)催化的磺酰苯胺的脱氢芳基化反应中,与C–N键相比,优选C–C键的功能。在本文中,据报道,通过soft离子对over离子的软硬酸碱(SHAB)控制,对磺酰苯胺进行了空前的N选择性芳基化。用碘(III)处理磺酰苯胺会导致形成亚硝酸根离子(软),其优先于联苯(软)而不是双苯甲醚(硬)与联苯反应生成C–N键。在室温下使用PhI和m CPBA原位生成碘(III)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号