当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Functionalization of a 1,4-Bis(trimethylsilyl)tetrasila-1,3-diene through the Selective Cleavage of Si(sp2)–Si(sp3) Bonds under Mild Reaction Conditions
Organometallics ( IF 2.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.organomet.7b00864 Naohiko Akasaka 1 , Kentaro Fujieda 1 , Eleonora Garoni 2, 3 , Kenji Kamada 2 , Hiroshi Matsui 4 , Masayoshi Nakano 4 , Takeaki Iwamoto 1
Organometallics ( IF 2.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.organomet.7b00864 Naohiko Akasaka 1 , Kentaro Fujieda 1 , Eleonora Garoni 2, 3 , Kenji Kamada 2 , Hiroshi Matsui 4 , Masayoshi Nakano 4 , Takeaki Iwamoto 1
Affiliation
|
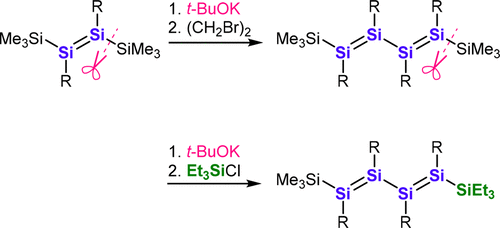
Although the oxidative coupling of disilenides, i.e., the disilicon analogues of vinyl anions, represents a promising route to extend the conjugation between Si═Si double bonds, previously reported synthetic routes to disilenides involve strongly reducing conditions. Herein, we report a novel synthetic route to disilenides from stable disilenes via the selective cleavage of Si(sp2)–Si(sp3) bonds under milder reaction conditions. Using this method, a 1,4-bis(trimethylsilyl)tetrasila-1,3-diene (5) was synthesized from the corresponding silyl-substituted disilene. Moreover, Et3Si-substituted tetrasila-1,3-diene 7 was synthesized via tetrasila-1,3-dien-1-ide 6, which is the first example of a functionalized tetrasila-1,3-diene.
中文翻译:

在温和的反应条件下,通过选择性裂解Si(sp 2)-Si(sp 3)键,合成1,4-双(三甲基甲硅烷基)四硅-1,3-二烯并使其功能化
尽管二硅化物,即乙烯基阴离子的二硅类似物的氧化偶联代表了扩展Si═Si双键之间共轭的有前途的途径,但是先前报道的二硅化物的合成途径涉及强烈的还原条件。在本文中,我们报告了在较温和的反应条件下通过选择性裂解Si(sp 2)-Si(sp 3)键从稳定的二硅萘烷中分离出二硅氢化物的新途径。使用该方法,由相应的甲硅烷基取代的二ilene合成了1,4-双(三甲基甲硅烷基)四硅-1,3-二烯(5)。此外,经由四硅-1,3-二烯-1-化物6合成了Et 3 Si-取代的四硅-1,3-二烯7。,这是官能化的四硅1,3-二烯的第一个实例。
更新日期:2018-01-06
中文翻译:

在温和的反应条件下,通过选择性裂解Si(sp 2)-Si(sp 3)键,合成1,4-双(三甲基甲硅烷基)四硅-1,3-二烯并使其功能化
尽管二硅化物,即乙烯基阴离子的二硅类似物的氧化偶联代表了扩展Si═Si双键之间共轭的有前途的途径,但是先前报道的二硅化物的合成途径涉及强烈的还原条件。在本文中,我们报告了在较温和的反应条件下通过选择性裂解Si(sp 2)-Si(sp 3)键从稳定的二硅萘烷中分离出二硅氢化物的新途径。使用该方法,由相应的甲硅烷基取代的二ilene合成了1,4-双(三甲基甲硅烷基)四硅-1,3-二烯(5)。此外,经由四硅-1,3-二烯-1-化物6合成了Et 3 Si-取代的四硅-1,3-二烯7。,这是官能化的四硅1,3-二烯的第一个实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号