当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Transformation and Diffusion Kinetics of V2O5 Electrode in Rechargeable Li and Mg Batteries: A First-Principle Study
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b11488 Renchao Xiao 1 , Jian Xie 1 , Ting Luo 1 , Liting Huang 1 , Yan Zhou 1 , Danmei Yu 1 , Changguo Chen 1 , Yuping Liu 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b11488 Renchao Xiao 1 , Jian Xie 1 , Ting Luo 1 , Liting Huang 1 , Yan Zhou 1 , Danmei Yu 1 , Changguo Chen 1 , Yuping Liu 1
Affiliation
|
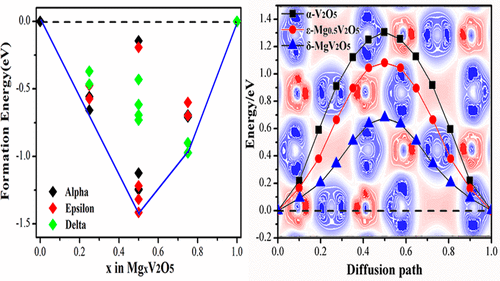
Using the density functional theory (DFT), first-principles computations were performed to investigate the intercalation site, phase transformation, electronic properties, and migration energy barrier of Li and Mg atoms in V2O5 to thoroughly illuminate the phase transformation and microscopic interaction as well as the migration mechanism of Li and Mg in V2O5. It is found that Li and Mg atoms prefer to locate at the site that is above, near the center of the quadrilateral composed of four V atoms. With the increase of the intercalation concentration (0 ≤ x ≤ 1), V2O5 undergoes a structural transformation from α-phase to ε-phase and δ-phase. Compared with ε-M0.5V2O5 (M = Li/Mg), the electronic conductivity of δ-MV2O5 (M = Li/Mg) is declined. On the basis of diffusion kinetics, Mg exhibits more difficulty in inserting and extracting in V2O5 than Li. This study can be useful for the further application of V2O5 in Mg ion batteries.
中文翻译:

锂和镁可充电电池中V 2 O 5电极的相变和扩散动力学:第一性原理研究
使用密度泛函理论(DFT),进行了第一性原理计算,以研究Li和Mg原子在V 2 O 5中的嵌入位置,相变,电子性质和迁移能垒,以彻底阐明相变和微观相互作用。以及Li和Mg在V 2 O 5中的迁移机理。发现Li和Mg原子更喜欢位于由四个V原子组成的四边形的中心上方的位置附近。与层夹浓度(0≤的增加X ≤1),V 2 ö 5经历了从α相到ε相和δ相的结构转变。与ε-M相比0.5 V 2 ø 5(M =锂/镁),δ-MV的电子传导性2 ø 5(M =锂/镁)的下降。根据扩散动力学,Mg在V 2 O 5中的插入和萃取比Li困难得多。这项研究对于镁离子电池中V 2 O 5的进一步应用可能是有用的。
更新日期:2018-01-16
中文翻译:

锂和镁可充电电池中V 2 O 5电极的相变和扩散动力学:第一性原理研究
使用密度泛函理论(DFT),进行了第一性原理计算,以研究Li和Mg原子在V 2 O 5中的嵌入位置,相变,电子性质和迁移能垒,以彻底阐明相变和微观相互作用。以及Li和Mg在V 2 O 5中的迁移机理。发现Li和Mg原子更喜欢位于由四个V原子组成的四边形的中心上方的位置附近。与层夹浓度(0≤的增加X ≤1),V 2 ö 5经历了从α相到ε相和δ相的结构转变。与ε-M相比0.5 V 2 ø 5(M =锂/镁),δ-MV的电子传导性2 ø 5(M =锂/镁)的下降。根据扩散动力学,Mg在V 2 O 5中的插入和萃取比Li困难得多。这项研究对于镁离子电池中V 2 O 5的进一步应用可能是有用的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号