当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Major Structural Change of the Homocitrate Ligand of Probable Importance for the Nitrogenase Mechanism
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02493 Per E. M. Siegbahn 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02493 Per E. M. Siegbahn 1
Affiliation
|
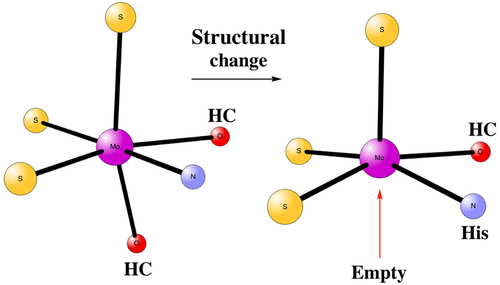
Mo-containing nitrogenase is the main enzyme that is able to take N2 from the air and form ammonia. The active-site cofactor of the enzyme, termed FeMoco, is unique in nature. It has seven Fe and one Mo atoms connected by S bridges, with a C atom in the center of the cofactor. Another unusual feature is that it has a large homocitrate ligand known to be of importance for catalysis. In the present computational study, the role of the homocitrate ligand is investigated. It is found that a large structural change, which makes MoIII five-coordinated, is energetically favorable in the more reduced states. This is of probable importance for the nitrogenase mechanism.
中文翻译:

硝化酶机制可能重要的同质配体的主要结构变化
含钼的固氮酶是能够从空气中吸收N 2并形成氨的主要酶。该酶的活性位点辅因子FeMoco在性质上是独特的。它有七个通过S桥连接的Fe和一个Mo原子,一个C原子位于辅因子的中心。另一个不寻常的特征是它具有大的均柠檬酸盐配体,已知该配体对催化很重要。在当前的计算研究中,研究了纯柠檬酸盐配体的作用。结果发现,较大的结构变化使Mo III处于五配位状态,在更大还原态下在能量上是有利的。这对于固氮酶机制可能很重要。
更新日期:2018-01-05
中文翻译:

硝化酶机制可能重要的同质配体的主要结构变化
含钼的固氮酶是能够从空气中吸收N 2并形成氨的主要酶。该酶的活性位点辅因子FeMoco在性质上是独特的。它有七个通过S桥连接的Fe和一个Mo原子,一个C原子位于辅因子的中心。另一个不寻常的特征是它具有大的均柠檬酸盐配体,已知该配体对催化很重要。在当前的计算研究中,研究了纯柠檬酸盐配体的作用。结果发现,较大的结构变化使Mo III处于五配位状态,在更大还原态下在能量上是有利的。这对于固氮酶机制可能很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号