当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Nickel Phosphide Carbonyl Nanoclusters: Synthesis, Structure, and Electrochemistry of [Ni11P(CO)18]3– and [H6–nNi31P4(CO)39]n− (n = 4 and 5)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02598 Chiara Capacci 1 , Iacopo Ciabatti 1 , Cristina Femoni 1 , Maria Carmela Iapalucci 1 , Tiziana Funaioli 2 , Stefano Zacchini 1 , Valerio Zanotti 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02598 Chiara Capacci 1 , Iacopo Ciabatti 1 , Cristina Femoni 1 , Maria Carmela Iapalucci 1 , Tiziana Funaioli 2 , Stefano Zacchini 1 , Valerio Zanotti 1
Affiliation
|
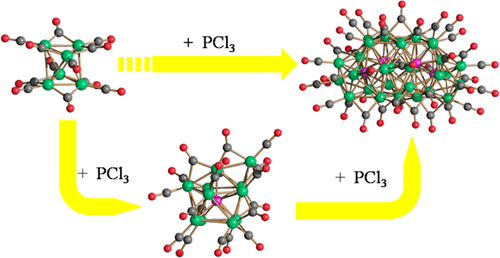
The reaction of [NEt4]2[Ni6(CO)12] in thf with 0.5 equiv of PCl3 affords the monophosphide [Ni11P(CO)18]3– that in turn further reacts with PCl3 resulting in the tetra-phosphide carbonyl cluster [HNi31P4(CO)39]5–. Alternatively, the latter can be obtained from the reaction of [NEt4]2[Ni6(CO)12] in thf with 0.8–0.9 equiv of PCl3. The [HNi31P4(CO)39]5– penta-anion is reversibly protonated by strong acids leading to the [H2Ni31P4(CO)39]4– tetra-anion, whereas deprotonation affords the [Ni31P4(CO)39]6– hexa-anion. The latter is reduced with Na/naphthalene yielding the [Ni31P4(CO)39]7– hepta-anion. In order to shed light on the polyhydride nature and redox behavior of these clusters, electrochemical and spectroelectrochemical studies were carried out on [Ni11P(CO)18]3–, [HNi31P4(CO)39]5–, and [H2Ni31P4(CO)39]4–. The reversible formation of the stable [Ni11P(CO)18]4– tetra-anion is demonstrated through the spectroelectrochemical investigation of [Ni11P(CO)18]3–. The redox changes of [HNi31P4(CO)39]5– show features of chemical reversibility and the vibrational spectra in the νCO region of the nine redox states of the cluster [HNi31P4(CO)39]n– (n = 3–11) are reported. The spectroelectrochemical investigation of [H2Ni31P4(CO)39]4– revealed the presence of three chemically reversible reduction processes, and the IR spectra of [H2Ni31P4(CO)39]n– (n = 4–7) have been recorded. The different spectroelectrochemical behavior of [HNi31P4(CO)39]5– and [H2Ni31P4(CO)39]4– support their formulations as polyhydrides. Unfortunately, all the attempts to directly confirm their poly hydrido nature by 1H NMR spectroscopy failed, as previously found for related large metal carbonyl clusters. Thus, the presence and number of hydride ligands have been based on the observed protonation/deprotonation reactions and the spectroelectrochemical experiments. The molecular structures of the new clusters have been determined by single-crystal X-ray analysis. These represent the first examples of structurally characterized molecular nickel carbonyl nanoclusters containing interstitial phosphide atoms.
中文翻译:

分子磷化镍羰基纳米簇的合成结构,和电化学的,[镍11 P(CO)18 ] 3-和[H 6- Ñ的Ni 31 P 4(CO)39 ] ñ - (Ñ = 4和5)
[NEt 4 ] 2 [Ni 6(CO)12 ]在thf中与0.5当量的PCl 3的反应提供了单磷[Ni 11 P(CO)18 ] 3-,该单磷化物进一步与PCl 3反应,从而得到四磷羰基簇[HNi 31 P 4(CO)39 ] 5–。或者,后者可以从[NEt 4 ] 2 [Ni 6(CO)12 ]在thf中与0.8-0.9当量的PCl 3反应获得。[HNi 31 P4(CO)39 ] 5–五价阴离子可被强酸质子化,产生[H 2 Ni 31 P 4(CO)39 ] 4–四阴离子,而去质子化可提供[Ni 31 P 4(CO)39 ] 6–六阴离子。后者用Na /萘还原,生成[Ni 31 P 4(CO)39 ] 7–七阴离子。为了阐明这些簇的多氢化合物性质和氧化还原行为,对[Ni 11P(CO)18 ] 3–,[HNi 31 P 4(CO)39 ] 5–和[H 2 Ni 31 P 4(CO)39 ] 4–。稳定的可逆的形成[镍11(CO)p 18 ] 4-四阴离子通过的光谱电化学调查[镍证明11 P(CO)18 ] 3-。[HNi 31 P 4(CO)39 ] 5–的氧化还原变化显示化学可逆性的功能并在ν振动光谱CO簇[HNI的九个氧化还原态的区域31 P 4(CO)39 ] N-(Ñ = 3-11)的报告。[H 2 Ni 31 P 4(CO)39 ] 4–的光谱电化学研究表明存在三个化学可逆的还原过程,[H 2 Ni 31 P 4(CO)39 ] n–(n= 4–7)已被记录。[HNi 31 P 4(CO)39 ] 5–和[H 2 Ni 31 P 4(CO)39 ] 4–的不同光谱电化学行为支持了它们的多氢化物配方。不幸的是,所有通过1直接证实其多氢化性质的尝试1 H NMR光谱法失败了,就像以前发现的有关大型羰基金属簇一样。因此,氢化物配体的存在和数量是基于观察到的质子化/去质子化反应和光谱电化学实验。通过单晶X射线分析确定了新簇的分子结构。这些代表了包含间隙磷原子的结构特征化的分子羰基镍纳米簇的第一个例子。
更新日期:2018-01-05
中文翻译:

分子磷化镍羰基纳米簇的合成结构,和电化学的,[镍11 P(CO)18 ] 3-和[H 6- Ñ的Ni 31 P 4(CO)39 ] ñ - (Ñ = 4和5)
[NEt 4 ] 2 [Ni 6(CO)12 ]在thf中与0.5当量的PCl 3的反应提供了单磷[Ni 11 P(CO)18 ] 3-,该单磷化物进一步与PCl 3反应,从而得到四磷羰基簇[HNi 31 P 4(CO)39 ] 5–。或者,后者可以从[NEt 4 ] 2 [Ni 6(CO)12 ]在thf中与0.8-0.9当量的PCl 3反应获得。[HNi 31 P4(CO)39 ] 5–五价阴离子可被强酸质子化,产生[H 2 Ni 31 P 4(CO)39 ] 4–四阴离子,而去质子化可提供[Ni 31 P 4(CO)39 ] 6–六阴离子。后者用Na /萘还原,生成[Ni 31 P 4(CO)39 ] 7–七阴离子。为了阐明这些簇的多氢化合物性质和氧化还原行为,对[Ni 11P(CO)18 ] 3–,[HNi 31 P 4(CO)39 ] 5–和[H 2 Ni 31 P 4(CO)39 ] 4–。稳定的可逆的形成[镍11(CO)p 18 ] 4-四阴离子通过的光谱电化学调查[镍证明11 P(CO)18 ] 3-。[HNi 31 P 4(CO)39 ] 5–的氧化还原变化显示化学可逆性的功能并在ν振动光谱CO簇[HNI的九个氧化还原态的区域31 P 4(CO)39 ] N-(Ñ = 3-11)的报告。[H 2 Ni 31 P 4(CO)39 ] 4–的光谱电化学研究表明存在三个化学可逆的还原过程,[H 2 Ni 31 P 4(CO)39 ] n–(n= 4–7)已被记录。[HNi 31 P 4(CO)39 ] 5–和[H 2 Ni 31 P 4(CO)39 ] 4–的不同光谱电化学行为支持了它们的多氢化物配方。不幸的是,所有通过1直接证实其多氢化性质的尝试1 H NMR光谱法失败了,就像以前发现的有关大型羰基金属簇一样。因此,氢化物配体的存在和数量是基于观察到的质子化/去质子化反应和光谱电化学实验。通过单晶X射线分析确定了新簇的分子结构。这些代表了包含间隙磷原子的结构特征化的分子羰基镍纳米簇的第一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号