Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.tet.2018.01.002 Chenggui Wu , Shanjian Mao , Guisheng Deng , Yongge Qiu
|
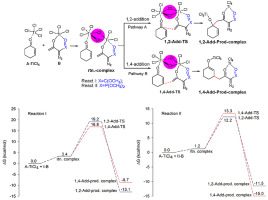
The regioselective addition mechanism of the Ti(IV) enolates derived from α-diazo-β-keto carbonyl compounds and α-diazo-β-keto phosphonates to conjugated enones has been studied on the basis of a hypothetical bridging chloride-controlled theory, by density functional theory (DFT), and experimentally. The DFT results indicate that, for the Ti(IV) enolate 3 derived from α-diazo-β-keto carbonyl compounds, the free energy of the bridging chloride-controlled 1,2-addition transition state is 2.4 kcal/mol higher than that of 1,4-addition, and the calculated enthalpies of 1,2-addition is 4.36 kcal/mol more than that of 1,4-addition. For the Ti(IV) enolate 4 derived from α-diazo-β-keto phosphonates, in contrary, the free energy of the bridging chloride-controlled 1,2-addition transition state is 1.1 kcal/mol lower than that of 1,4-addition, and the calculated enthalpy of 1,2-addition is 3.46 kcal/mol less than that of 1,4-addition. Our findings demonstrate that the nucleophilic addition of these Ti(IV) enolates to conjugated enones was carried out not only kinetically but also irreversibly for the first time.
中文翻译:

钛烯酸酯向共轭烯酮的区域选择性加成:基于实验和DFT研究的机理的新见解
在假定桥接氯化物控制理论的基础上,研究了由α-重氮-β-酮羰基化合物和α-重氮-β-酮膦酸酯衍生的Ti(IV)烯醇盐对共轭烯酮的区域选择性加成机理。密度泛函理论(DFT)和实验。DFT结果表明,对于由α-重氮-β-酮羰基化合物衍生的Ti(IV)烯醇盐3,桥连氯控制的1,2-加成过渡态的自由能比其高2.4 kcal / mol。 1,4-加成的计算焓,比1,4-加成的计算焓为4.36 kcal / mol。对于Ti(IV)烯酸酯4相反,由α-重氮-β-酮膦酸酯衍生而来的是,桥接氯化物控制的1,2-加成过渡态的自由能比1,4-加成的自由能低1.1 kcal / mol,并且计算出的焓为1,2加成比1,4加成少3.46 kcal / mol。我们的发现表明,这些Ti(IV)烯酸酯向共轭烯酮的亲核加成反应不仅是动力学的,而且是不可逆的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号