当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of the marine alkaloids 6-oxofascaplysin, fascaplysin and their derivatives
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.tetlet.2018.01.023 Maxim E. Zhidkov , Alexey V. Kantemirov , Alexey V. Koisevnikov , Alexander N. Andin , Alexandra S. Kuzmich
中文翻译:

海洋生物碱6-氧天冬青素,法西林素及其衍生物的合成
更新日期:2018-01-06
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.tetlet.2018.01.023 Maxim E. Zhidkov , Alexey V. Kantemirov , Alexey V. Koisevnikov , Alexander N. Andin , Alexandra S. Kuzmich
|
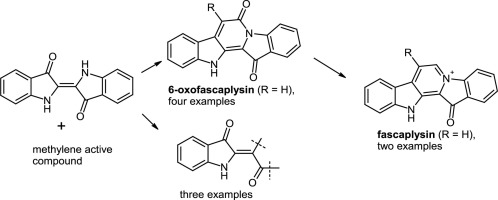
A simple approach towards the pyrido[1,2-a:3,4-b′]diindole system via the reaction of indigo with methylene active compounds was used for the syntheses of the marine alkaloids 6-oxofascaplysin, fascaplysin, and their derivatives. It was also demonstrated that the reaction with ketones led to indigo decomposition and the formation of isatin derivatives. The derivative of fascaplysin with a phenyl substituent at C-7 demonstrated 2–3 times greater inhibitory activity against selected cancer cell lines than fascaplysin.
中文翻译:

海洋生物碱6-氧天冬青素,法西林素及其衍生物的合成
通过靛蓝与亚甲基活性化合物反应制得吡啶并[1,2- a:3,4- b ']二吲哚系统的一种简单方法是用于合成海洋生物碱6-oxofascaplysin,fascaplysin及其衍生物。还证明了与酮的反应导致靛蓝分解和靛红衍生物的形成。具有在C-7处有一个苯基取代基的fascaplysin衍生物对Fascaplysin的抑制作用是对fascaplysin的2-3倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号