当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
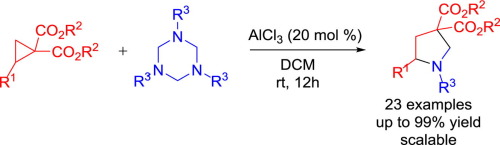
Accessing substituted pyrrolidines via formal [3+2] cycloaddition of 1,3,5-triazinanes and donor-acceptor cyclopropanes
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.tetlet.2018.01.016 Zhi-Yao Chu , Na Li , Dan Liang , Zheng-Hui Li , Yong-Sheng Zheng , Ji-Kai Liu
中文翻译:

通过1,3,5-三嗪并有供体-受体环丙烷的[3 + 2]环加成反应获得取代的吡咯烷
更新日期:2018-01-06
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.tetlet.2018.01.016 Zhi-Yao Chu , Na Li , Dan Liang , Zheng-Hui Li , Yong-Sheng Zheng , Ji-Kai Liu
|
The formal [3+2] cycloaddition of 1,3,5-triaryl-1,3,5-triazinanes with donor-acceptor cyclopropanes has been found to provide pyrrolidines in good to excellent yields under mild reaction conditions. Preliminary mechanistic investigation indicates that this formal [3+2] cycloaddition reaction proceeds through competing SN1 and SN2 pathways.
中文翻译:

通过1,3,5-三嗪并有供体-受体环丙烷的[3 + 2]环加成反应获得取代的吡咯烷
已经发现,在温和的反应条件下,将1,3,5-三芳基-1,3,5-三嗪酮与供体-受体环丙烷进行正式的[3 + 2]环加成反应可提供吡咯烷类化合物。初步的机理研究表明,这种正式的[3 + 2]环加成反应是通过竞争性的S N 1和S N 2途径进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号