Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-01-05 , DOI: 10.1016/j.jfluchem.2018.01.003 Wenbo Dong , Xin Yang , Jianbo Cheng , Wenzuo Li , Qingzhong Li
|
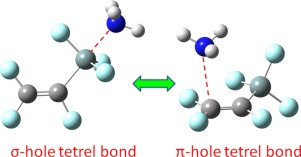
An ab initio calculation has been performed for the complexes of F2CCFTF3 (T
C, Si, and Ge) with three Lewis bases including H2CO, H2O, and NH3. Most complexes are stabilized by tetrel bonds together with weak hydrogen bonds. The Lewis bases can be introduced to the σ-hole and π-hole of F2C
CFTF3, but the σ-hole bonded complexes are preferential for F2C
CFTF3 (T
Si and Ge), while the π-hole bonded complexes are favorable for F2C
CFCF3. Hybridization of the carbon atom adjoined with the SiF3 group strengthens the σ-hole tetrel bond in the order sp3 < sp2 < sp, similar to hydrogen and halogen bonds. The strength of σ-hole tetrel bond is also dependent on the number, position, and conformation of substituents. In addition, solvents have a prominent enhancing effect on the strength of σ-hole tetrel bond with about 0.1 Å shortening of the Si⋯N distance in water.
中文翻译:

涉及F 2 C
已经对F 2 C CFTF 3(TC
,Si和Ge)与三个路易斯碱(包括H 2 CO,H 2 O和NH 3)的配合物进行了从头计算。多数配合物都由锡特尔键和弱氢键稳定。可以将Lewis碱引入F 2 C
CFTF 3的σ孔和π孔中,但σ孔键合的络合物优先于F 2 C
CFTF 3(T
Si和Ge),而π孔键合络合物对F 2 C
CFCF 3有利。与SiF 3基团相邻的碳原子的杂化以sp 3 <sp 2 <sp的顺序增强了σ-孔t形键,类似于氢键和卤素键。σ-孔t形键的强度还取决于取代基的数量,位置和构象。此外,溶剂对σ-孔t固键的强度具有显着的增强作用,使水中的Si⋯N距离缩短约0.1Å。











































 京公网安备 11010802027423号
京公网安备 11010802027423号