Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2018-01-06 , DOI: 10.1016/j.jhazmat.2018.01.011 Jizi Wu , Dan Huang , Xingmei Liu , Jun Meng , Caixian Tang , Jianming Xu
|
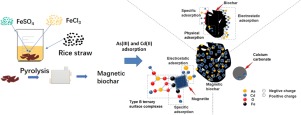
A novel calcium-based magnetic biochar (Ca-MBC), made by pyrolyzing the mixture of rice straw, iron oxide (Fe3O4) and calcium carbonate (CaCO3), was developed in this study for remediation of co-pollution of arsenic and cadmium. Characteristics of the material showed that Fe3O4 and CaCO3 were adhered on the surface of biochar. The experiments on the effects of pH, adsorption kinetics and isotherm revealed that the Ca-MBC had a great ability to adsorb arsenic and cadmium within 0.5 h for cadmium and 12 h for arsenic with a maximum adsorption capacity of 6.34 and 10.07 mg g-1, respectively, and that the adsorption of both metals was pH-dependent from 2 to 12 with an optimal pH of pH 5. The mechanism of co-adsorption of Cd(II) and As(III) included both competitive and synergistic effects. The presence of As(III) enhanced Cd(II) adsorption by 3-16% while Cd(II) addition suppressed As(III) adsorption by 15-33%. The synergistic effects on As(III) and Cd(II) adsorption had resulted from the electrostatic interaction and the formation of type B ternary surface complexes. These new insights provide valuable information for the application of Ca-MBC as a potential adsorbent in treatment of water contaminated with As(III) and Cd(II).
中文翻译:

新型钙基磁性生物炭对水系统中As(III)和Cd(II)共污染的修复及其机理
本研究开发了一种新型的基于钙的磁性生物炭(Ca-MBC),该材料通过热解稻草,氧化铁(Fe 3 O 4)和碳酸钙(CaCO 3)的混合物而制得,可用于共污染食品。砷和镉。材料的特性表明Fe 3 O 4和CaCO 3附着在生物炭的表面上。对pH,吸附动力学和等温线影响的实验表明,Ca-MBC具有强大的吸附能力,在0.5 h内对镉和12 h内砷和镉的吸附能力最大,最大吸附量为6.34和10.07 mg g -1。,并且两种金属的吸附均在2至12的pH值范围内变化,最佳pH值为5。Cd(II)和As(III)的共吸附机理包括竞争和协同作用。As(III)的存在将Cd(II)的吸附提高了3-16%,而Cd(II)的添加将As(III)的吸附抑制了15-33%。静电相互作用和B型三元表面配合物的形成对As(III)和Cd(II)的吸附具有协同作用。这些新见解为将Ca-MBC用作潜在吸附剂处理被As(III)和Cd(II)污染的水提供了有价值的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号