当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies on the Catalytic Synthesis of BN Heterocycles (1H-2,1-Benzazaboroles) at Ruthenium
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b03461 Marion Beguerie 1, 2 , Chiara Dinoi 3, 4 , Iker del Rosal 3, 4 , Charly Faradji 1, 2 , Gilles Alcaraz 1, 2 , Laure Vendier 1, 2 , Sylviane Sabo-Etienne 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b03461 Marion Beguerie 1, 2 , Chiara Dinoi 3, 4 , Iker del Rosal 3, 4 , Charly Faradji 1, 2 , Gilles Alcaraz 1, 2 , Laure Vendier 1, 2 , Sylviane Sabo-Etienne 1, 2
Affiliation
|
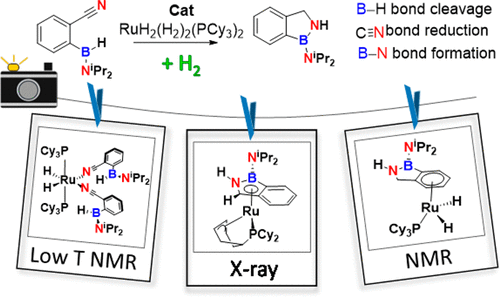
We had recently disclosed a catalyzed transformation toward the synthesis of BN molecules under an H2 atmosphere under mild conditions. We now report an in-depth mechanistic study to understand how a substrate featuring two different functional groups, C≡N and B–H, namely the 2-cyanophenyl(amino)borane HB(NiPr2)C6H4(CN) (2), can be transformed into the BN heterocycle 1H-2,1-benzazaborole (3). Such a complex transformation has direct links with three key important processes: hydrogenation of nitriles, hydroboration of polar bonds, and B–N bond formation. A combination of in situ monitoring of the catalytic reaction, stoichiometric experiments, and variable-temperature multinuclear NMR and DFT studies allowed us to decipher the catalytic cycle. We show that the catalyst precursor [RuH2(η2-H2)2(PCy3)2] (1) is regenerated at the end of the transformation. We intercepted the transformation of the starting substrate 2, in the form of a 1H-2,1-benzazaborolyl ligand coordinated to the metal center by the formed BN cycle. The corresponding benzazaboryl complex [Ru{(η5-C(H)N(H)B(NiPr2)(C6H4)}{(η3-C6H8)PCy2}] (9) was independently prepared and fully characterized by X-ray diffraction and multinuclear NMR. We also showed that complex 9 undergoes stepwise hydrogenation, followed by haptotropic rearrangement before release of the final product 3 and regeneration of the catalyst precursor 1. We were able to provide a fairly good view of the activation of the C≡N and B–H bonds. So far, it appears that nitrile hydroboration with metal hydrides starts with nitrile reduction but subsequent steps are highly dependent on the system. In our case, after the first hydrogen transfer to the nitrile, a boron–nitrogen interaction is highly favored, B–H bond cleavage occurring at a later stage. This field needs further investigation for promising developments of BN molecules. Prospects on reactions involving at least two different intramolecular reactive functions should be encouraged for future development in catalysis.
中文翻译:

钌催化合成BN杂环(1 H -2,1-苯并氮杂硼烷)的机理研究
我们最近公开了在温和条件下在H 2气氛下催化合成BN分子的转化过程。现在,我们报告了一项深入的机理研究,以了解底物如何具有两个不同的官能团C≡N和B–H,即2-氰基苯基(氨基)硼烷HB(N i Pr 2)C 6 H 4(CN )(2),可以转化为BN杂环1 H -2,1-benzazaborole(3)。如此复杂的转变与三个关键的重要过程有直接联系:腈的氢化,极性键的硼氢化和B–N键的形成。催化反应的原位监测,化学计量学实验以及变温多核NMR和DFT研究相结合,使我们能够破译催化循环。我们表明,在催化剂前体[期RuH 2(η 2 -H 2)2(PCY 3)2 ](1)在变换的结束再生。我们以1 H的形式截获了起始底物2的转变-2,1-苯并氮杂硼烯丙基配体通过形成的BN循环配位至金属中心。benzazaboryl复杂相应的[Ru {(η 5 -C(H)N(H)B(N我镨2)(C 6 H ^ 4)} {(η 3 -C 6 ħ 8)PCY 2 }](9)该化合物是独立制备的,并通过X射线衍射和多核NMR进行了全面表征,还显示了络合物9经历了逐步加氢,随后发生触觉重排,然后释放出最终产物3并再生了催化剂前体1。。我们能够很好地了解C≡N和B–H键的激活。到目前为止,看来用金属氢化物进行腈加氢硼化是从腈还原开始的,但随后的步骤高度依赖于该系统。在我们的案例中,在氢第一次转移到腈中之后,硼与氮的相互作用受到了极大的支持,B-H键的裂解发生在随后的阶段。该领域需要进一步研究,以开发有希望的BN分子。应鼓励涉及至少两种不同分子内反应功能的反应的前景,以促进催化的未来发展。
更新日期:2018-01-05
中文翻译:

钌催化合成BN杂环(1 H -2,1-苯并氮杂硼烷)的机理研究
我们最近公开了在温和条件下在H 2气氛下催化合成BN分子的转化过程。现在,我们报告了一项深入的机理研究,以了解底物如何具有两个不同的官能团C≡N和B–H,即2-氰基苯基(氨基)硼烷HB(N i Pr 2)C 6 H 4(CN )(2),可以转化为BN杂环1 H -2,1-benzazaborole(3)。如此复杂的转变与三个关键的重要过程有直接联系:腈的氢化,极性键的硼氢化和B–N键的形成。催化反应的原位监测,化学计量学实验以及变温多核NMR和DFT研究相结合,使我们能够破译催化循环。我们表明,在催化剂前体[期RuH 2(η 2 -H 2)2(PCY 3)2 ](1)在变换的结束再生。我们以1 H的形式截获了起始底物2的转变-2,1-苯并氮杂硼烯丙基配体通过形成的BN循环配位至金属中心。benzazaboryl复杂相应的[Ru {(η 5 -C(H)N(H)B(N我镨2)(C 6 H ^ 4)} {(η 3 -C 6 ħ 8)PCY 2 }](9)该化合物是独立制备的,并通过X射线衍射和多核NMR进行了全面表征,还显示了络合物9经历了逐步加氢,随后发生触觉重排,然后释放出最终产物3并再生了催化剂前体1。。我们能够很好地了解C≡N和B–H键的激活。到目前为止,看来用金属氢化物进行腈加氢硼化是从腈还原开始的,但随后的步骤高度依赖于该系统。在我们的案例中,在氢第一次转移到腈中之后,硼与氮的相互作用受到了极大的支持,B-H键的裂解发生在随后的阶段。该领域需要进一步研究,以开发有希望的BN分子。应鼓励涉及至少两种不同分子内反应功能的反应的前景,以促进催化的未来发展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号