Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-05 , DOI: 10.1016/j.tetlet.2018.01.010 Khursheed Ansari , Ali Mohd Lone , Wajaht Amin Shah , Jagdamba Singh , I.R. Siddiqui
|
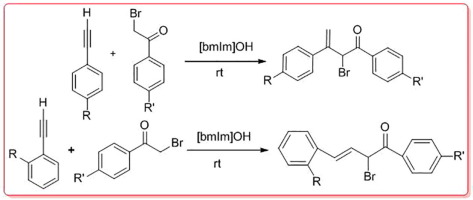
Effective methodology that activates selectively either end of a carbon–carbon triple bond requires key challenge of differentiating between the multitude of CC bonds present in complex organic molecule. The synthetic strategy exploited the electronic biases within the substrate and successfully achieved site–selective [bmIm]OH catalyzed C
C bond functionalization under mild reaction conditions. This resulted in C-2-selective addition of phenacyl bromide on p-substituted phenyl acetylene and C-1 selective addition on the o-substituted phenyl acetylene leading to C
C bond formation. The reaction proceeded smoothly with excellent yield under ambient conditions. This report demonstrates the progress on the catalytic activity of recyclable [bmIm]OH for selective C
C bond formation.
中文翻译:

有效地选择性激活碳-碳三键任一端的方法学要求对区分复杂有机分子中存在的多个C C键的关键挑战。合成策略利用了底物中的电子偏压,并
在温和的反应条件下成功实现了位点选择性[bmIm] OH催化的C C键功能化。这导致对C-2选择性加成苯甲酰甲基溴的p取代的苯基乙炔和在C-1选择性加成ö取代的苯基乙炔导致至C
C键形成。在环境条件下,反应顺利进行并具有优异的产率。该报告证明了可循环利用的[bmIm] OH对选择性C
C键形成的催化活性的研究进展。







































 京公网安备 11010802027423号
京公网安备 11010802027423号