Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Selective Etherification and Transetherification Reactions Using Alcohols
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acsomega.7b01705 Prakash Kumar Sahoo 1 , Suhas Shahaji Gawali 1 , Chidambaram Gunanathan 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acsomega.7b01705 Prakash Kumar Sahoo 1 , Suhas Shahaji Gawali 1 , Chidambaram Gunanathan 1
Affiliation
|
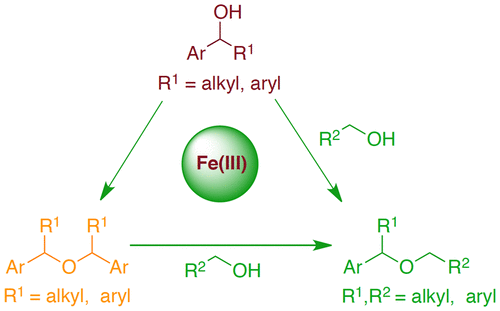
Simple and readily available iron(III) triflate turned out to be a cheap, environmentally benign, and efficient catalyst for the direct etherification of alcohols. The use of ammonium chloride as an additive (5 mol %, 1 equiv relative to catalyst) suppressed the side reactions completely and ensured the selective ether formation even on challenging substrates containing electron-donating substituents. This method allows the selective synthesis of symmetrical ethers from benzylic secondary alcohols and unsymmetrical ethers directly from secondary and primary alcohols. Moreover, transetherification of symmetrical ethers using primary alcohols is attained. The reaction progress of symmetrical ether and unsymmetrical ether formation followed zero-order and first-order kinetics, respectively. Electron paramagnetic resonance (EPR) measurements of the reaction mixture and simple iron(III) triflate clearly indicated that oxidation state of the metal center remains same throughout the catalysis. Mechanistic studies confirmed that the unsymmetrical ether formation occurs via the in situ formed symmetrical ethers.
中文翻译:

使用醇进行铁催化的选择性醚化和醚交换反应
结果表明,简单易用的三氟甲磺酸铁(III)是一种廉价,对环境无害且对醇进行直接醚化的有效催化剂。使用氯化铵作为添加剂(5 mol%,相对于催化剂1当量)完全抑制了副反应,即使在具有给电子取代基的具有挑战性的底物上也确保了选择性醚的形成。该方法允许由苄基仲醇选择性合成对称醚,而直接由仲醇和伯醇选择性合成不对称醚。此外,使用伯醇实现了对称醚的醚交换。对称醚和不对称醚形成的反应进程分别遵循零级和一级动力学。反应混合物和简单的三氟甲磺酸铁的电子顺磁共振(EPR)测量清楚地表明,在整个催化过程中,金属中心的氧化态保持相同。机理研究证实,不对称醚的形成是通过原位形成的对称醚发生的。
更新日期:2018-01-05
中文翻译:

使用醇进行铁催化的选择性醚化和醚交换反应
结果表明,简单易用的三氟甲磺酸铁(III)是一种廉价,对环境无害且对醇进行直接醚化的有效催化剂。使用氯化铵作为添加剂(5 mol%,相对于催化剂1当量)完全抑制了副反应,即使在具有给电子取代基的具有挑战性的底物上也确保了选择性醚的形成。该方法允许由苄基仲醇选择性合成对称醚,而直接由仲醇和伯醇选择性合成不对称醚。此外,使用伯醇实现了对称醚的醚交换。对称醚和不对称醚形成的反应进程分别遵循零级和一级动力学。反应混合物和简单的三氟甲磺酸铁的电子顺磁共振(EPR)测量清楚地表明,在整个催化过程中,金属中心的氧化态保持相同。机理研究证实,不对称醚的形成是通过原位形成的对称醚发生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号