当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Does the Catalyst Affect the Reaction Pathway? DFT Analysis of the Mechanism and Selectivity in the 1,6-Diyne Ester Cycloisomerization
Organometallics ( IF 2.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.organomet.7b00813 Yunhe Li 1 , Peng-Cheng Tu 1 , Lin Zhou 1 , Alexander M. Kirillov 2 , Ran Fang 1 , Lizi Yang 1
Organometallics ( IF 2.5 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.organomet.7b00813 Yunhe Li 1 , Peng-Cheng Tu 1 , Lin Zhou 1 , Alexander M. Kirillov 2 , Ran Fang 1 , Lizi Yang 1
Affiliation
|
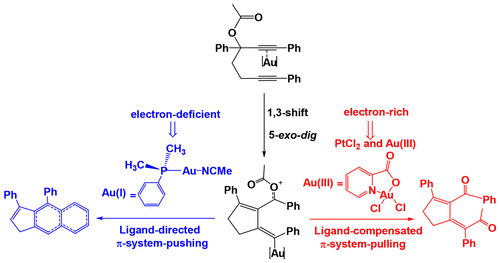
The present study reports a detailed theoretical analysis of the mechanistic and chemoselectivity features in 1,6-diyne ester cycloisomerization. The energy profiles for three different catalysts, namely, [AuI(PPhMe2)(NCMe)]+, [AuIII(Cl)2(pic)] (pic = 2-picolinate), and PtCl2, were investigated. The DFT calculations reveal that all of these catalysts entail similar 1,3-acyloxy migration and 5-exo-dig cyclization steps, whereas completely distinct reaction pathways are observed after the formation of the putative vinyl metal complex intermediates. In the [AuI(PPhMe2)(NCMe)]+ system, the configuration of the phosphine ligand can explain the exclusive chemoselectivity of the Friedel–Crafts reaction over the 1,5-acyl migration. On the other hand, in the [AuIII(Cl)2(pic)] and PtCl2 systems, the 1,5-acyl migration is assisted by the chloride ligand, offering an alternative mechanism that can justify a reasonable activation barrier and the corresponding stereochemical feature in the reaction. Moreover, the [AuI(PPhMe2)(NCMe)]+ complex with soft and carbophilic character represents an electron-deficient catalyst with a linear structure; it is particularly unsuitable for the 1,5-acyl migration. In contrast, the [AuIII(Cl)2(pic)] catalyst reveals a distorted-square-planar geometry that satisfies the condition to form a square-planar moiety with an acyl functionality. Thus, the obtained theoretical results not only well rationalize the experimental observations but provide insights into the details of the 1,5-acyl migration.
中文翻译:

催化剂如何影响反应途径?DFT分析1,6-二炔酯环异构化的机理和选择性
本研究报告详细的理论分析机制和化学选择性特征在1,6-二炔酯环异构化。研究了三种不同催化剂,即[Au I(PPhMe 2)(NCMe)] +,[Au III(Cl)2(pic)](pic = 2-picolinate)和PtCl 2的能谱。DFT计算表明,所有这些催化剂都需要相似的1,3-酰氧基迁移和5 -exo-dig环化步骤,而在推定的乙烯基金属络合物中间体形成后观察到完全不同的反应途径。在[Au I(PPhMe 2)(NCMe)]中+在系统中,膦配体的构型可以解释Friedel-Crafts反应在1,5-酰基迁移过程中的唯一化学选择性。另一方面,在[Au III(Cl)2(pic)]和PtCl 2体系中,1,5-酰基的迁移由氯化物配体协助,提供了另一种机制,该机制可以证明合理的活化屏障和反应中相应的立体化学特征。另外,具有柔软且具有亲碳性的[Au I(PPhMe 2)(NCMe)] +络合物表示具有线性结构的缺电子催化剂。它特别不适用于1,5-酰基迁移。相反,[Au III(Cl)[图2(图片)]的催化剂显示出满足满足形成具有酰基官能度的方平面部分的条件的方平面畸变的几何形状。因此,获得的理论结果不仅很好地使实验观察合理化,而且提供了对1,5-酰基迁移细节的见解。
更新日期:2018-01-05
中文翻译:

催化剂如何影响反应途径?DFT分析1,6-二炔酯环异构化的机理和选择性
本研究报告详细的理论分析机制和化学选择性特征在1,6-二炔酯环异构化。研究了三种不同催化剂,即[Au I(PPhMe 2)(NCMe)] +,[Au III(Cl)2(pic)](pic = 2-picolinate)和PtCl 2的能谱。DFT计算表明,所有这些催化剂都需要相似的1,3-酰氧基迁移和5 -exo-dig环化步骤,而在推定的乙烯基金属络合物中间体形成后观察到完全不同的反应途径。在[Au I(PPhMe 2)(NCMe)]中+在系统中,膦配体的构型可以解释Friedel-Crafts反应在1,5-酰基迁移过程中的唯一化学选择性。另一方面,在[Au III(Cl)2(pic)]和PtCl 2体系中,1,5-酰基的迁移由氯化物配体协助,提供了另一种机制,该机制可以证明合理的活化屏障和反应中相应的立体化学特征。另外,具有柔软且具有亲碳性的[Au I(PPhMe 2)(NCMe)] +络合物表示具有线性结构的缺电子催化剂。它特别不适用于1,5-酰基迁移。相反,[Au III(Cl)[图2(图片)]的催化剂显示出满足满足形成具有酰基官能度的方平面部分的条件的方平面畸变的几何形状。因此,获得的理论结果不仅很好地使实验观察合理化,而且提供了对1,5-酰基迁移细节的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号