当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of a Heterocyclic Nitrogen-Donor Group on the Coordination of Trivalent Actinides and Lanthanides by Aminopolycarboxylate Complexants
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02792 Travis S. Grimes 1 , Colt R. Heathman 1 , Santa Jansone-Popova 2 , Alexander S. Ivanov 2 , Santanu Roy 2 , Vyacheslav S. Bryantsev 2 , Peter R. Zalupski 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02792 Travis S. Grimes 1 , Colt R. Heathman 1 , Santa Jansone-Popova 2 , Alexander S. Ivanov 2 , Santanu Roy 2 , Vyacheslav S. Bryantsev 2 , Peter R. Zalupski 1
Affiliation
|
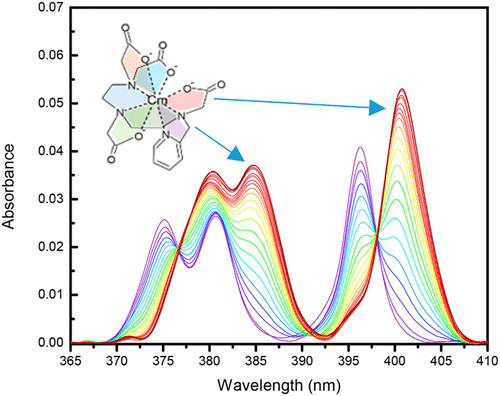
The novel metal chelator N-2-(pyridylmethyl)diethylenetriamine-N,N′,N″,N″-tetraacetic acid (DTTA-PyM) was designed to replace a single oxygen-donor acetate group of the well-known aminopolycarboxylate complexant diethylenetriamine-N,N,N′,N″,N″-pentaacetic acid (DTPA) with a nitrogen-donor 2-pyridylmethyl. Potentiometric, spectroscopic, computational, and radioisotope distribution methods show distinct differences for the 4f and 5f coordination environments and enhanced actinide binding due to the nitrogen-bearing heterocyclic moiety. The Am3+, Cm3+, and Ln3+ complexation studies for DTTA-PyM reveal an enhanced preference, relative to DTPA, for trivalent actinide binding. Fluorescence studies indicate no changes to the octadentate coordination of trivalent curium, while evidence of heptadentate complexation of trivalent europium is found in mixtures containing EuHL(aq) complexes at the same aqueous acidity. The denticity change observed for Eu3+ suggests that complex protonation occurs on the pyridyl nitrogen. Formation of the CmHL(aq) complex is likely due to the protonation of an available carboxylate group because the carbonyl oxygen can maintain octadentate coordination through a rotation. The observed suppressed protonation of the pyridyl nitrogen in the curium complexes may be attributed to stronger trivalent actinide binding by DTTA-PyM. Density functional theory calculations indicate that added stabilization of the actinide complexes with DTTA-PyM may originate from π-back-bonding interactions between singly occupied 5f orbitals of Am3+ and the pyridyl nitrogen. The differences between the stabilities of trivalent actinide chelates (Am3+, Cm3+) and trivalent lanthanide chelates (La3+–Lu3+) are observed in liquid–liquid extraction systems, yielding unprecedented 4f/5f differentiation when using DTTA-PyM as an aqueous holdback reagent. In addition, the enhanced nitrogen-donor softness of the new DTTA-PyM chelator was perturbed by adding a fluorine onto the pyridine group. The comparative characterization of N-(3-fluoro-2-pyridylmethyl)diethylenetriamine-N,N′,N″,N″-tetraacetic acid (DTTA-3-F-PyM) showed subdued 4f/5f differentiation due to the presence of this electron-withdrawing group.
中文翻译:

杂环氮-供体基团对氨基多羧酸络合物配位三价Act系元素和镧系元素配位的影响
新颖的金属螯合剂Ñ -2-(吡啶基甲基)二亚乙基Ñ,Ñ ',Ñ “,Ñ ” -四乙酸(DTTA-PYM)被设计用来替换公知的aminopolycarboxylate络合剂二亚乙基三单个氧供体乙酸酯基-具有氮供体2-吡啶基甲基的N,N,N ′,N ″ ,N ″,N ″-五乙酸(DTPA)。电位,光谱,计算和放射性同位素分布方法在4 f和5 f上显示出明显的差异含氮杂环部分,从而改善了配位环境并增强了act系元素的结合。DTTA-PyM的Am 3+,Cm 3+和Ln 3+络合研究显示,相对于DTPA,三价act系元素结合的偏好增强。荧光研究表明,三价cur的八齿配位没有变化,而在含水酸度相同的含EuHL (aq)络合物的混合物中发现了三价he的七齿络合的证据。对于Eu 3+观察到的密度变化表明,在吡啶基氮上发生了复杂的质子化。CmHL (aq)的形成由于羰基氧可通过旋转保持八齿配位,因此可能是由于可用的羧酸酯基团的质子化而形成的络合物。cur络合物中吡啶氮原子的抑制质子化可能归因于DTTA-PyM与三价act系元素的结合更强。密度泛函理论计算表明the系化合物与DTTA-PyM的附加稳定性可能源自Am 3+单独占据的5 f轨道与吡啶氮之间的π反向键相互作用。三价act系元素螯合物(Am 3+,Cm 3+)和三价镧系元素螯合物(La 3+ –Lu 3+)在液-液萃取系统中观察到,当使用DTTA-PyM作为含水保留试剂时,可产生空前的4 f / 5 f差异。此外,通过在吡啶基团上添加氟,可以扰乱新的DTTA-PyM螯合剂的氮供体柔软性。的比较表征ñ - (3-氟-2-吡啶基甲基)二亚乙基Ñ,Ñ ',Ñ “,Ñ ” -四乙酸(DTTA-3-F-PYM)显示柔和4 ˚F / 5 ˚F分化因该吸电子基团的存在。
更新日期:2018-01-05
中文翻译:

杂环氮-供体基团对氨基多羧酸络合物配位三价Act系元素和镧系元素配位的影响
新颖的金属螯合剂Ñ -2-(吡啶基甲基)二亚乙基Ñ,Ñ ',Ñ “,Ñ ” -四乙酸(DTTA-PYM)被设计用来替换公知的aminopolycarboxylate络合剂二亚乙基三单个氧供体乙酸酯基-具有氮供体2-吡啶基甲基的N,N,N ′,N ″ ,N ″,N ″-五乙酸(DTPA)。电位,光谱,计算和放射性同位素分布方法在4 f和5 f上显示出明显的差异含氮杂环部分,从而改善了配位环境并增强了act系元素的结合。DTTA-PyM的Am 3+,Cm 3+和Ln 3+络合研究显示,相对于DTPA,三价act系元素结合的偏好增强。荧光研究表明,三价cur的八齿配位没有变化,而在含水酸度相同的含EuHL (aq)络合物的混合物中发现了三价he的七齿络合的证据。对于Eu 3+观察到的密度变化表明,在吡啶基氮上发生了复杂的质子化。CmHL (aq)的形成由于羰基氧可通过旋转保持八齿配位,因此可能是由于可用的羧酸酯基团的质子化而形成的络合物。cur络合物中吡啶氮原子的抑制质子化可能归因于DTTA-PyM与三价act系元素的结合更强。密度泛函理论计算表明the系化合物与DTTA-PyM的附加稳定性可能源自Am 3+单独占据的5 f轨道与吡啶氮之间的π反向键相互作用。三价act系元素螯合物(Am 3+,Cm 3+)和三价镧系元素螯合物(La 3+ –Lu 3+)在液-液萃取系统中观察到,当使用DTTA-PyM作为含水保留试剂时,可产生空前的4 f / 5 f差异。此外,通过在吡啶基团上添加氟,可以扰乱新的DTTA-PyM螯合剂的氮供体柔软性。的比较表征ñ - (3-氟-2-吡啶基甲基)二亚乙基Ñ,Ñ ',Ñ “,Ñ ” -四乙酸(DTTA-3-F-PYM)显示柔和4 ˚F / 5 ˚F分化因该吸电子基团的存在。



























 京公网安备 11010802027423号
京公网安备 11010802027423号