当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tailor-Making Fluorescent Hyaluronic Acid Microgels via Combining Microfluidics and Photoclick Chemistry for Sustained and Localized Delivery of Herceptin in Tumors
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acsami.7b15832 Jing Chen 1 , Ke Huang 1 , Qijun Chen 1 , Chao Deng 1 , Jian Zhang 1 , Zhiyuan Zhong 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acsami.7b15832 Jing Chen 1 , Ke Huang 1 , Qijun Chen 1 , Chao Deng 1 , Jian Zhang 1 , Zhiyuan Zhong 1
Affiliation
|
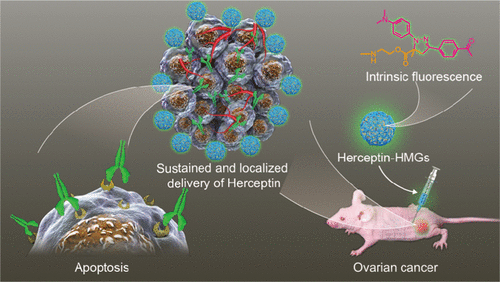
Antibody therapeutics, though representing a most used biomedicine, suffers from poor in vivo stability, rapid degradation, and frequent injections. Here, we report that fluorescent hyaluronic acid microgels (HMGs) tailor-made by combining microfluidics and “tetrazole–alkene” photoclick chemistry enable sustained and localized delivery of Herceptin in ovarian tumors. HMGs were obtained with a defined size (25–50 μm), narrow size distribution, high stability, and strong green fluorescence. Notably, HMGs exhibited a remarkably high loading of proteins such as Herceptin and IgG with a loading efficiency exceeding 90% at a theoretical protein-loading content of 30 wt %. In vitro protein release experiments revealed a sustained and hyaluronidase (HAase)-dependent release of Herceptin from HMGs, in which 80.6% of Herceptin was released at 1 U/mL HAase in 10 days. The released Herceptin maintained its secondary structure and antitumor activity. In vivo imaging results demonstrated obviously better tumoral retention for Cy5-labeled Herceptin-loaded HMGs following subcutaneous (sc) injection than for the free-protein counterpart. Interestingly, sc injection of the Herceptin-loaded HMGs into SKOV-3 human ovarian tumor-bearing nude mice at a dose of 30 mg Herceptin equiv/kg induced nearly complete tumor suppression, which was significantly more effective than the sc or systemic injection of free Herceptin. These tailor-made fluorescent HMGs appeared as a robust injectable platform for sustained and localized delivery of therapeutic proteins.
中文翻译:

通过结合微流控和Photoclick化学量身定制的荧光透明质酸微凝胶在肿瘤中持续和局部递送赫赛汀
抗体疗法虽然代表了最常用的生物医学,但其体内稳定性差,降解速度快以及经常注射会遭受痛苦。在这里,我们报道通过结合微流控技术和“四唑-烯烃”光点击化学方法量身定制的荧光透明质酸微凝胶(HMG),可以在卵巢肿瘤中持续和局部递送赫赛汀。获得的HMG具有确定的尺寸(25–50μm),尺寸分布窄,高稳定性和强绿色荧光。值得注意的是,HMGs在理论蛋白质负载量为30 wt%的情况下,表现出很高的蛋白质负载量,例如赫赛汀和IgG,负载效率超过90%。体外蛋白质释放实验表明,HMG持续且透明质酸酶(HAase)依赖于HMG释放,其中80。在10天内,以1 U / mL HAase释放了6%的Herceptin。释放的赫赛汀保持其二级结构和抗肿瘤活性。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。以30 mg Herceptin equiv / kg的剂量将荷塞汀负载的HMG皮下注射到SKOV-3人卵巢荷瘤裸鼠中,几乎可以完全抑制肿瘤,这比皮下注射或全身性注射游离赫赛汀有效得多。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。以30 mg Herceptin equiv / kg的剂量将荷塞汀负载的HMG皮下注射到SKOV-3人卵巢荷瘤裸鼠中,几乎可以完全抑制肿瘤,这比皮下注射或全身性注射游离赫赛汀有效得多。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。
更新日期:2018-01-16
中文翻译:

通过结合微流控和Photoclick化学量身定制的荧光透明质酸微凝胶在肿瘤中持续和局部递送赫赛汀
抗体疗法虽然代表了最常用的生物医学,但其体内稳定性差,降解速度快以及经常注射会遭受痛苦。在这里,我们报道通过结合微流控技术和“四唑-烯烃”光点击化学方法量身定制的荧光透明质酸微凝胶(HMG),可以在卵巢肿瘤中持续和局部递送赫赛汀。获得的HMG具有确定的尺寸(25–50μm),尺寸分布窄,高稳定性和强绿色荧光。值得注意的是,HMGs在理论蛋白质负载量为30 wt%的情况下,表现出很高的蛋白质负载量,例如赫赛汀和IgG,负载效率超过90%。体外蛋白质释放实验表明,HMG持续且透明质酸酶(HAase)依赖于HMG释放,其中80。在10天内,以1 U / mL HAase释放了6%的Herceptin。释放的赫赛汀保持其二级结构和抗肿瘤活性。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。体内成像结果表明,皮下(sc)注射后,Cy5标记的Herceptin负载的HMG的肿瘤保留明显优于游离蛋白对应物。有趣的是,将荷塞汀负载的HMG以30 mg Herceptin equiv / kg的剂量皮下注射到SKOV-3人卵巢荷瘤裸鼠中,诱导了几乎完全的肿瘤抑制,这比皮下注射或全身注射游离HMG更为有效。赫赛汀。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。以30 mg Herceptin equiv / kg的剂量将荷塞汀负载的HMG皮下注射到SKOV-3人卵巢荷瘤裸鼠中,几乎可以完全抑制肿瘤,这比皮下注射或全身性注射游离赫赛汀有效得多。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。以30 mg Herceptin equiv / kg的剂量将荷塞汀负载的HMG皮下注射到SKOV-3人卵巢荷瘤裸鼠中,几乎可以完全抑制肿瘤,这比皮下注射或全身性注射游离赫赛汀有效得多。这些量身定制的荧光HMG似乎是用于治疗性蛋白质持续和局部递送的强大的可注射平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号