当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heck Reaction of Electronically Diverse Tertiary Alkyl Halides
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.orglett.7b03591 Daria Kurandina 1 , Mónica Rivas 1 , Maxim Radzhabov 1 , Vladimir Gevorgyan 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.orglett.7b03591 Daria Kurandina 1 , Mónica Rivas 1 , Maxim Radzhabov 1 , Vladimir Gevorgyan 1
Affiliation
|
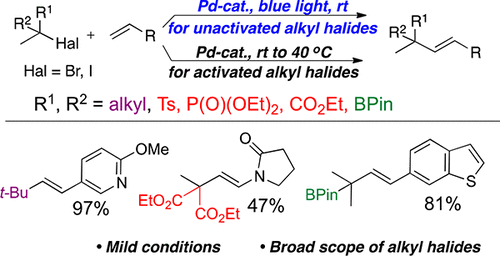
The efficient Pd-catalyzed Heck reaction of diverse tertiary alkyl halides with alkenes has been developed. Unactivated tertiary alkyl halides efficiently react at room temperature under visible light irradiation with no exogenous photosensitizers required. For activated tertiary alkyl halides, the same catalytic system works well without light. These methods offer a general access to electronically diverse alkenes possessing quaternary and functionalized tertiary allylic carbon centers. The substituents at these centers include alkyl-, carbalkoxy-, tosyl-, phosphonyl-, and boronate groups. It was also shown that the end-game mechanism of this transformation may vary depending on the type of the substrates used.
中文翻译:

电子多样的叔烷基卤化物的 Heck 反应
多种叔烷基卤化物与烯烃的高效 Pd 催化 Heck 反应已被开发出来。未活化的叔烷基卤化物可在室温下、可见光照射下有效反应,无需外源光敏剂。对于活化的叔烷基卤化物,相同的催化系统在没有光的情况下也能很好地工作。这些方法提供了获得具有四级和官能化叔烯丙基碳中心的电子多样化烯烃的一般途径。这些中心的取代基包括烷基-、烷氧基-、甲苯磺酰基-、膦酰基-和硼酸酯基团。研究还表明,这种转化的最终机制可能会根据所用基材的类型而有所不同。
更新日期:2018-01-05
中文翻译:

电子多样的叔烷基卤化物的 Heck 反应
多种叔烷基卤化物与烯烃的高效 Pd 催化 Heck 反应已被开发出来。未活化的叔烷基卤化物可在室温下、可见光照射下有效反应,无需外源光敏剂。对于活化的叔烷基卤化物,相同的催化系统在没有光的情况下也能很好地工作。这些方法提供了获得具有四级和官能化叔烯丙基碳中心的电子多样化烯烃的一般途径。这些中心的取代基包括烷基-、烷氧基-、甲苯磺酰基-、膦酰基-和硼酸酯基团。研究还表明,这种转化的最终机制可能会根据所用基材的类型而有所不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号