当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Cleavage of Inert Aryl C–N Bonds in N-Aryl Amides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02880 Zhiguo Zhang 1, 2 , Dan Zheng 1 , Yameng Wan 1 , Guisheng Zhang 1 , Jingjing Bi 1 , Qingfeng Liu 1 , Tongxin Liu 1 , Lei Shi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02880 Zhiguo Zhang 1, 2 , Dan Zheng 1 , Yameng Wan 1 , Guisheng Zhang 1 , Jingjing Bi 1 , Qingfeng Liu 1 , Tongxin Liu 1 , Lei Shi 1
Affiliation
|
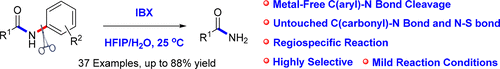
A highly selective, IBX-promoted reaction has been developed for the oxidative cleavage of inert C(aryl)–N bonds on secondary amides while leaving the C(carbonyl)–N bond unchanged. This metal-free reaction proceeds under mild conditions (HFIP/H2O, 25 °C), providing facile access to various useful primary amides, some of which would be otherwise unattainable using conventional aminolysis and hydrolysis approaches.
中文翻译:

惰性芳基C-N键的选择性裂解在Ñ -芳基酰胺
已经开发出一种高度选择性的IBX促进的反应,用于氧化裂解仲酰胺上的惰性C(芳基)-N键,同时保持C(羰基)-N键不变。这种无金属的反应在温和的条件下(HFIP / H 2 O,25°C)进行,可以轻松获得各种有用的伯酰胺,而使用常规的氨解和水解方法则无法实现其中的某些。
更新日期:2018-01-17
中文翻译:

惰性芳基C-N键的选择性裂解在Ñ -芳基酰胺
已经开发出一种高度选择性的IBX促进的反应,用于氧化裂解仲酰胺上的惰性C(芳基)-N键,同时保持C(羰基)-N键不变。这种无金属的反应在温和的条件下(HFIP / H 2 O,25°C)进行,可以轻松获得各种有用的伯酰胺,而使用常规的氨解和水解方法则无法实现其中的某些。










































 京公网安备 11010802027423号
京公网安备 11010802027423号