当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unbiased Molecular Dynamics of 11 min Timescale Drug Unbinding Reveals Transition State Stabilizing Interactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-05 , DOI: 10.1021/jacs.7b08572 Samuel D Lotz 1 , Alex Dickson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-05 , DOI: 10.1021/jacs.7b08572 Samuel D Lotz 1 , Alex Dickson 1
Affiliation
|
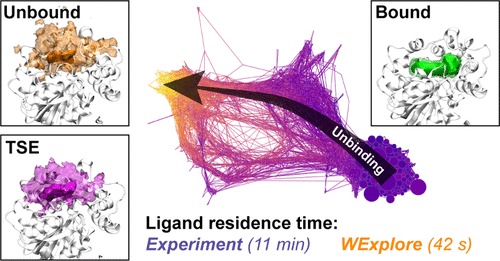
Ligand (un)binding kinetics is being recognized as a determinant of drug specificity and efficacy in an increasing number of systems. However, the calculation of kinetics and the simulation of drug unbinding is more difficult than computing thermodynamic quantities, such as binding free energies. Here we present the first full simulations of an unbinding process at pharmacologically relevant time scales (11 min), without the use of biasing forces, detailed prior knowledge, or specialized processors using the weighted ensemble based algorithm, WExplore. These simulations show the inhibitor TPPU unbinding from its enzyme target soluble epoxide hydrolase, which is a clinically relevant target that has attracted interest in kinetics optimization in order to increase efficacy. We make use of conformation space networks that allow us to conceptualize unbinding not just as a linear process, but as a network of interconnected states that connect the bound and unbound states. This allows us to visualize patterns in hydrogen-bonding, solvation, and nonequilibrium free energies, without projection onto progress coordinates. The topology and layout of the network reveal multiple unbinding pathways, and other rare events, such as the reversal of ligand orientation within the binding site. Furthermore, we make a prediction of the transition state ensemble, using transition path theory, and identify protein-ligand interactions which are stabilizing to the transition state. Additionally, we uncover trends in ligand and binding site solvation that corroborate experimental evidence from more classical structure kinetics relationships and generate new questions as to the role of drug modifications in kinetics optimization. Finally, from only 6 μs of simulation time we observed 75 unbinding events from which we calculate a residence time of 42 s, and a standard error range of 23 to 280 s. This nearly encompasses the experimental residence time 11 min (660 s). In addition to the insights to sEH inhibitor unbinding, this study shows that simulations of complex processes on timescales as long as minutes are becoming feasible for more researchers to perform.
中文翻译:

11 分钟时间尺度药物解绑的无偏分子动力学揭示了过渡态稳定相互作用
在越来越多的系统中,配体(非)结合动力学被认为是药物特异性和功效的决定因素。然而,动力学的计算和药物解结合的模拟比计算热力学量更困难,例如结合自由能。在这里,我们展示了在药理学相关时间尺度(11 分钟)上对解绑过程的首次完整模拟,不使用偏置力、详细的先验知识或使用基于加权集成的算法 WExplore 的专用处理器。这些模拟显示抑制剂 TPPU 与其酶靶标可溶性环氧化物水解酶解除结合,这是一个临床相关的靶标,引起了对动力学优化的兴趣,以提高功效。我们利用构象空间网络,使我们不仅可以将解除绑定概念化为一个线性过程,还可以将其概念化为连接绑定和未绑定状态的互连状态网络。这使我们能够将氢键、溶剂化和非平衡自由能的模式可视化,而无需投影到进程坐标上。网络的拓扑结构和布局揭示了多种解结合途径和其他罕见事件,例如结合位点内配体方向的逆转。此外,我们使用过渡路径理论对过渡态集合进行预测,并确定稳定到过渡态的蛋白质-配体相互作用。此外,我们发现了配体和结合位点溶剂化的趋势,这些趋势证实了来自更经典结构动力学关系的实验证据,并产生了关于药物修饰在动力学优化中的作用的新问题。最后,仅从 6 μs 的模拟时间我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间和 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。从仅 6 μs 的模拟时间,我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间,以及 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。从仅 6 μs 的模拟时间,我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间,以及 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。
更新日期:2018-01-05
中文翻译:

11 分钟时间尺度药物解绑的无偏分子动力学揭示了过渡态稳定相互作用
在越来越多的系统中,配体(非)结合动力学被认为是药物特异性和功效的决定因素。然而,动力学的计算和药物解结合的模拟比计算热力学量更困难,例如结合自由能。在这里,我们展示了在药理学相关时间尺度(11 分钟)上对解绑过程的首次完整模拟,不使用偏置力、详细的先验知识或使用基于加权集成的算法 WExplore 的专用处理器。这些模拟显示抑制剂 TPPU 与其酶靶标可溶性环氧化物水解酶解除结合,这是一个临床相关的靶标,引起了对动力学优化的兴趣,以提高功效。我们利用构象空间网络,使我们不仅可以将解除绑定概念化为一个线性过程,还可以将其概念化为连接绑定和未绑定状态的互连状态网络。这使我们能够将氢键、溶剂化和非平衡自由能的模式可视化,而无需投影到进程坐标上。网络的拓扑结构和布局揭示了多种解结合途径和其他罕见事件,例如结合位点内配体方向的逆转。此外,我们使用过渡路径理论对过渡态集合进行预测,并确定稳定到过渡态的蛋白质-配体相互作用。此外,我们发现了配体和结合位点溶剂化的趋势,这些趋势证实了来自更经典结构动力学关系的实验证据,并产生了关于药物修饰在动力学优化中的作用的新问题。最后,仅从 6 μs 的模拟时间我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间和 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。从仅 6 μs 的模拟时间,我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间,以及 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。从仅 6 μs 的模拟时间,我们观察到 75 个解结合事件,从中我们计算出 42 秒的停留时间,以及 23 到 280 秒的标准误差范围。这几乎涵盖了实验停留时间 11 分钟(660 秒)。除了对 sEH 抑制剂解除结合的见解之外,这项研究还表明,对于更多的研究人员来说,只要几分钟就可以模拟复杂过程的时间尺度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号