当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic [3 + 2] cycloaddition of oxindole-based azomethine ylides with 3-nitrochromenes: a facile approach to enantioenriched polycyclic spirooxindole-chromane adducts†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7ob03051g Shiqi Wu 1, 2, 3, 4 , Guodong Zhu 2, 4, 5, 6, 7 , Shiqiang Wei 2, 4, 5, 6, 7 , Hongbo Chen 1, 2, 3, 4 , Jingping Qu 2, 4, 5, 6, 7 , Baomin Wang 2, 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7ob03051g Shiqi Wu 1, 2, 3, 4 , Guodong Zhu 2, 4, 5, 6, 7 , Shiqiang Wei 2, 4, 5, 6, 7 , Hongbo Chen 1, 2, 3, 4 , Jingping Qu 2, 4, 5, 6, 7 , Baomin Wang 2, 4, 5, 6, 7
Affiliation
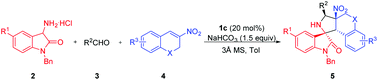
|
An organocatalytic asymmetric [3 + 2] cycloaddition of oxindole-based azomethine ylides with 3-nitro-2H-chromenes has been developed. This reaction provides a facile approach to densely functionalized polycyclic spirooxindole-chromane adducts featuring four contiguous stereogenic centers, including two tetrasubstituted carbon centers. The products were obtained in high yields with good to excellent stereoselectivities (up to 99% yields, 96% ee and >20 : 1 dr). In addition, the spiro[pyrrolidine-2,3′-oxindole]-chromane adducts could be readily derivatized via simple oxidation and reduction treatment. A dual activation working model to illuminate the stereochemical course of the [3 + 2] cycloaddition event is proposed.
中文翻译:

含3-硝基色酮的基于吲哚的甲亚胺基团的有机催化[3 + 2]环加成:对映体富集的多环螺并恶吲哚-苯并二氢苯并二氢吡喃加合物的简便方法†
已经开发了基于氧吲哚的偶氮甲亚胺与3-硝基-2 H-色烯的有机催化不对称[3 + 2]环加成反应。该反应为以四个连续的立体中心,包括两个四取代的碳中心为特征的致密官能化的多环螺氧基吲哚-苯并二氢呋喃加合物提供了简便的方法。以高收率获得具有良好至优异的立体选择性的产物(高达99%的收率,96%的ee和> 20:1dr)。另外,可以通过简单的氧化和还原处理容易地衍生化螺[吡咯烷-2,3'-羟吲哚]-苯并二氢吡喃加合物。提出了双重激活的工作模型,以阐明[3 + 2]环加成事件的立体化学过程。
更新日期:2018-01-05
中文翻译:

含3-硝基色酮的基于吲哚的甲亚胺基团的有机催化[3 + 2]环加成:对映体富集的多环螺并恶吲哚-苯并二氢苯并二氢吡喃加合物的简便方法†
已经开发了基于氧吲哚的偶氮甲亚胺与3-硝基-2 H-色烯的有机催化不对称[3 + 2]环加成反应。该反应为以四个连续的立体中心,包括两个四取代的碳中心为特征的致密官能化的多环螺氧基吲哚-苯并二氢呋喃加合物提供了简便的方法。以高收率获得具有良好至优异的立体选择性的产物(高达99%的收率,96%的ee和> 20:1dr)。另外,可以通过简单的氧化和还原处理容易地衍生化螺[吡咯烷-2,3'-羟吲哚]-苯并二氢吡喃加合物。提出了双重激活的工作模型,以阐明[3 + 2]环加成事件的立体化学过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号