当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed chemoselective alcoholysis of N-acyloxazolidinones
Green Chemistry ( IF 9.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7gc03534a Pei-Qiang Huang 1, 2, 3, 4, 5 , Hui Geng 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7gc03534a Pei-Qiang Huang 1, 2, 3, 4, 5 , Hui Geng 1, 2, 3, 4, 5
Affiliation
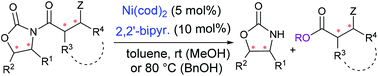
|
Although N-acyloxazolidinone-based (catalytic) asymmetric synthetic methodologies occupy an important position in modern organic synthesis, the catalytic cleavage of a chiral auxiliary remains underdeveloped. We report the Ni(cod)2/bipyr.-catalyzed alcoholysis of N-acyloxazolidinones to deliver esters. The reaction is broad in scope for both N-acyloxazolidinone substrates and alcohol nucleophiles, and displays good functional group tolerance and excellent chemoselectivity. A gram-scale methanolysis allowed the enantioselective synthesis of the C22–C26 segment of a close analogue of the potent immunosuppressant agent FK506.
中文翻译:

Ni催化的N-酰基恶唑烷酮的化学选择性醇解
尽管基于N-酰基氧杂唑烷酮的(催化)不对称合成方法在现代有机合成中占有重要地位,但手性助剂的催化裂解仍未开发。我们报告了N(酰基)xx恶唑烷酮的Ni(cod)2 /联吡啶催化的醇解反应以递送酯。该反应对于N-酰基氧杂唑烷酮底物和醇亲核试剂都具有广泛的范围,并且显示出良好的官能团耐受性和优异的化学选择性。克级的甲醇分解作用可以使强效免疫抑制剂FK506的紧密类似物的C22–C26片段进行对映选择性合成。
更新日期:2018-02-06
中文翻译:

Ni催化的N-酰基恶唑烷酮的化学选择性醇解
尽管基于N-酰基氧杂唑烷酮的(催化)不对称合成方法在现代有机合成中占有重要地位,但手性助剂的催化裂解仍未开发。我们报告了N(酰基)xx恶唑烷酮的Ni(cod)2 /联吡啶催化的醇解反应以递送酯。该反应对于N-酰基氧杂唑烷酮底物和醇亲核试剂都具有广泛的范围,并且显示出良好的官能团耐受性和优异的化学选择性。克级的甲醇分解作用可以使强效免疫抑制剂FK506的紧密类似物的C22–C26片段进行对映选择性合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号