当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal alkyls programmed to generate metal alkylidenes by α-H abstraction: prognosis from NMR chemical shift†
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7sc05039a Christopher P Gordon 1 , Keishi Yamamoto 1 , Keith Searles 1 , Satoru Shirase 1, 2 , Richard A Andersen 3 , Odile Eisenstein 4, 5 , Christophe Copéret 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7sc05039a Christopher P Gordon 1 , Keishi Yamamoto 1 , Keith Searles 1 , Satoru Shirase 1, 2 , Richard A Andersen 3 , Odile Eisenstein 4, 5 , Christophe Copéret 1
Affiliation
|
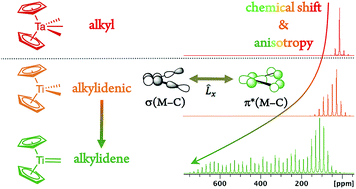
Metal alkylidenes, which are key organometallic intermediates in reactions such as olefination or alkene and alkane metathesis, are typically generated from metal dialkyl compounds [M](CH2R)2 that show distinctively deshielded chemical shifts for their α-carbons. Experimental solid-state NMR measurements combined with DFT/ZORA calculations and a chemical shift tensor analysis reveal that this remarkable deshielding originates from an empty metal d-orbital oriented in the M–Cα–Cα′ plane, interacting with the Cα p-orbital lying in the same plane. This π-type interaction inscribes some alkylidene character into Cα that favors alkylidene generation via α-H abstraction. The extent of the deshielding and the anisotropy of the alkyl chemical shift tensors distinguishes [M](CH2R)2 compounds that form alkylidenes from those that do not, relating the reactivity to molecular orbitals of the respective molecules. The α-carbon chemical shifts and tensor orientations thus predict the reactivity of metal alkyl compounds towards alkylidene generation.
中文翻译:

烷基金属被编程以通过 α-H 抽象生成金属亚烷基:NMR 化学位移的预测†
亚烷基金属是烯烃化或烯烃和烷烃复分解等反应中的关键有机金属中间体,通常由金属二烷基化合物 [M](CH 2 R) 2生成,其 α-碳具有明显的去屏蔽化学位移。实验固态 NMR 测量与 DFT/ZORA 计算和化学位移张量分析相结合,表明这种显着的去屏蔽源于一个空的金属 d 轨道,取向在 M-C α -C α′平面上,与 C α p相互作用- 位于同一平面的轨道。这种 π 型相互作用将一些亚烷基特征刻录到 C α中,这有利于通过α-H 抽象。烷基化学位移张量的去屏蔽程度和各向异性将形成亚烷基的[M](CH 2 R) 2化合物与不形成亚烷基的化合物区分开来,将反应性与相应分子的分子轨道相关联。因此,α-碳化学位移和张量取向预测了金属烷基化合物对亚烷基生成的反应性。
更新日期:2018-01-05
中文翻译:

烷基金属被编程以通过 α-H 抽象生成金属亚烷基:NMR 化学位移的预测†
亚烷基金属是烯烃化或烯烃和烷烃复分解等反应中的关键有机金属中间体,通常由金属二烷基化合物 [M](CH 2 R) 2生成,其 α-碳具有明显的去屏蔽化学位移。实验固态 NMR 测量与 DFT/ZORA 计算和化学位移张量分析相结合,表明这种显着的去屏蔽源于一个空的金属 d 轨道,取向在 M-C α -C α′平面上,与 C α p相互作用- 位于同一平面的轨道。这种 π 型相互作用将一些亚烷基特征刻录到 C α中,这有利于通过α-H 抽象。烷基化学位移张量的去屏蔽程度和各向异性将形成亚烷基的[M](CH 2 R) 2化合物与不形成亚烷基的化合物区分开来,将反应性与相应分子的分子轨道相关联。因此,α-碳化学位移和张量取向预测了金属烷基化合物对亚烷基生成的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号