当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-catalyzed [5+1] annulation of δ-sulfonamido-substituted enones with N-sulfonylimines: a facile synthesis of tetrahydropyridines†
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7sc04515h Leijie Zhou 1 , Chunhao Yuan 1 , Yuan Zeng 1 , Honglei Liu 1 , Chang Wang 1 , Xing Gao 1 , Qijun Wang 1 , Cheng Zhang 1 , Hongchao Guo 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7sc04515h Leijie Zhou 1 , Chunhao Yuan 1 , Yuan Zeng 1 , Honglei Liu 1 , Chang Wang 1 , Xing Gao 1 , Qijun Wang 1 , Cheng Zhang 1 , Hongchao Guo 1
Affiliation
|
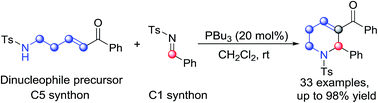
Phosphine-catalyzed [5+1] annulation of δ-sulfonamido-substituted enones with N-sulfonylimines for the synthesis of 1,2,3,6-tetrahydropyridines is developed. The reaction proceeds smoothly under mild reaction conditions to give the annulation products in moderate to excellent yields. Mechanistic exploration of this new annulation shows that the δ-sulfonamido-substituted enone and the N-sulfonylimine serve as C5 and C1 synthons to furnish the annulation, respectively. Using chiral phosphine as the catalyst, an asymmetric variant of the model reaction gave the chiral product in up to 73% ee.
中文翻译:

膦催化 δ-磺酰氨基取代烯酮与 N-磺酰亚胺的 [5+1] 环化:四氢吡啶的简便合成†
开发了膦催化的 δ-磺酰胺基取代烯酮与N-磺酰亚胺的 [5+1] 成环反应,用于合成 1,2,3,6-四氢吡啶。该反应在温和的反应条件下顺利进行,以中等至优异的收率得到环化产物。对这种新环化的机理探索表明,δ-磺酰胺基取代的烯酮和N-磺酰亚胺分别作为C5和C1合成子来提供环化。使用手性膦作为催化剂,模型反应的不对称变体产生了高达 73% ee 的手性产物。
更新日期:2018-01-05
中文翻译:

膦催化 δ-磺酰氨基取代烯酮与 N-磺酰亚胺的 [5+1] 环化:四氢吡啶的简便合成†
开发了膦催化的 δ-磺酰胺基取代烯酮与N-磺酰亚胺的 [5+1] 成环反应,用于合成 1,2,3,6-四氢吡啶。该反应在温和的反应条件下顺利进行,以中等至优异的收率得到环化产物。对这种新环化的机理探索表明,δ-磺酰胺基取代的烯酮和N-磺酰亚胺分别作为C5和C1合成子来提供环化。使用手性膦作为催化剂,模型反应的不对称变体产生了高达 73% ee 的手性产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号