Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.jcat.2017.12.008 Meiqing Shen , Yun Zhang , Jianqiang Wang , Chen Wang , Jun Wang
|
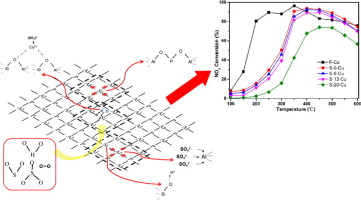
To reveal the nature of SO3 poisoning of Cu/SAPO-34 selective catalytic reduction (SCR) catalysts, CHA structure, copper species, and NOx conversion were probed. The Cu/SAPO-34 catalyst was treated with different ratios of SO3 to SOx (0, 6, 13, and 20%) at 250 °C. The breakage of SiO(H)
Al bonds takes place and leads to aluminum sulfate formation upon SOx sulfation. More isolated Cu2+ transform to copper sulfate with an increment of the SO3/SOx ratio. Catalysts poisoned with SOx show lower NOx conversion at 150–300 °C, the reason for which is a reduced number of isolated Cu2+ sites, as their turnover frequencies (TOFs) are identical. The loss of isolated Cu2+ and Si
O(H)
Al bonds is responsible for the inferior activity above 300 °C. The presence of SO3 cannot be ignored because of the irreversible reaction with Cu/SAPO-34 when diesel oxidation catalysts are applied upstream of the SCR.
中文翻译:

Cu / SAPO-34 SCR催化剂上SO 3中毒的性质
为了揭示Cu / SAPO-34选择性催化还原(SCR)催化剂的SO 3中毒性质,对CHA结构,铜种类和NO x转化率进行了研究。在250°C下以不同比例的SO 3与SO x(0、6、13和20%)处理Cu / SAPO-34催化剂。发生Si O(H)
Al键断裂,并导致SO x硫酸化形成硫酸铝。随着SO 3 / SO x比的增加,更多的孤立的Cu 2+转化为硫酸铜。被SO x毒化的催化剂显示出较低的NO x在150–300°C时发生转化,其原因是孤立的Cu 2+位点数量减少,因为它们的周转频率(TOF)相同。分离出的Cu 2+和Si
O(H)
Al键的丧失是造成高于300°C的劣活性的原因。当在SCR的上游使用柴油氧化催化剂时,由于与Cu / SAPO-34发生不可逆反应,因此不能忽略SO 3的存在。



























 京公网安备 11010802027423号
京公网安备 11010802027423号