当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accelerated Stress Test of Pt/C Nanoparticles in an Interface with an Anion-Exchange Membrane—An Identical-Location Transmission Electron Microscopy Study
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acscatal.7b04055 Clémence Lafforgue 1 , Marian Chatenet 1, 2 , Laetitia Dubau 1 , Dario R. Dekel 3, 4
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acscatal.7b04055 Clémence Lafforgue 1 , Marian Chatenet 1, 2 , Laetitia Dubau 1 , Dario R. Dekel 3, 4
Affiliation
|
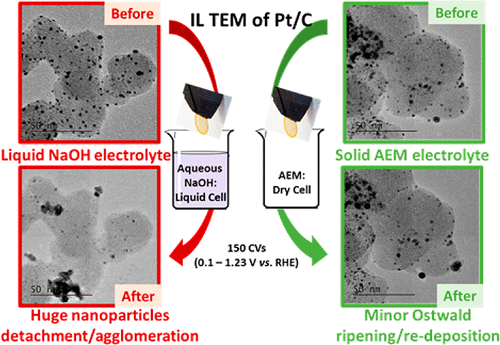
The durability of a state-of-the-art Pt/C electrocatalyst was assessed by accelerated stress test (AST) procedures conducted in liquid alkaline electrolyte (0.1 M NaOH) and in solid anion exchange polymer electrolyte using a “dry cell”, i.e. in absence of liquid electrolyte. In a liquid environment, the positive and negative vertex potential values have a great influence on the extent and on the magnitude of the degradations: the loss of electrochemical surface area observed for a wide potential range (0.1 < E < 1.23 V vs RHE) is ascribed to detachment of the Pt nanoparticles from their support. The Pt nanoparticles assist the local corrosion of the carbon support material, eventually yielding solid alkali–metal carbonates that mechanically expel them from their support. Such corrosion is linked to the propensity of the Pt nanoparticles to (i) accept carbon surface groups (COad-like species) when their surface is free of oxides (“reduced” metal state, for E < 0.6 V vs RHE) and then to (ii) electro-oxidize the COad species into CO2 in the well-known Langmuir–Hinshelwood CO-stripping reaction, possible if OHad species do form (“oxidized” metal state, for E > 0.6 V vs RHE). As a result, when the AST is performed between 0.1 < E < 0.6 V vs RHE or between 0.6 < E < 1.23 V vs RHE, i.e. when the Pt nanoparticles are either mostly reduced or oxidized, respectively, the degradation processes at stake are less intense and different: Ostwald ripening proceeds in the former case and Pt nanoparticle agglomeration in the latter. In contrast to the case of liquid electrolyte, when the most severe AST (0.1 < E < 1.23 V vs RHE) is performed in the dry cell, the magnitude and main mechanisms of degradation significantly change. Because there is no excess water to dissolve the Ptz+ species formed by corrosion of the Pt nanoparticles, 3D Ostwald ripening and local redeposition on existing particles become more likely: the anion-exchange ionomer better traps the Ptz+ species and prevent their diffusion away from the active layer. In addition, the absence of free alkali metal cation avoids the precipitation of solid carbonates, and therefore, the detachment of the Pt nanoparticles from their support is not favored. This shows that the degradation processes of a given electrocatalyst not only depends on its nature but also on the vertex potential values scanned in the AST and, importantly, on the nature of the electrolyte medium investigated. Finally, the very dramatic degradations experienced in liquid electrolyte for Pt/C nanoparticles are somewhat mitigated in solid alkaline electrolyte, which harbors hope to develop durable AEM-based fuel cells and electrolyzers.
中文翻译:

阴离子交换膜界面中Pt / C纳米粒子的加速应力测试-相同位置的透射电子显微镜研究
通过在液体碱性电解质(0.1 M NaOH)和固体阴离子交换聚合物电解质中使用“干电池”(即“干电池”)进行的加速应力测试(AST)程序,评估了最新技术的Pt / C电催化剂的耐久性。在没有液体电解质的情况下。在液体环境中,正和负顶点电势值对降解的程度和程度有很大影响:在宽电势范围内观察到的电化学表面积损失(0.1 < E相对于RHE小于1.23 V)归因于Pt纳米颗粒从其载体上脱离。Pt纳米颗粒有助于碳载体材料的局部腐蚀,最终产生固体碱金属碳酸盐,从而将其从载体上机械排出。此类腐蚀与Pt纳米颗粒倾向于(i)当碳表面基团的表面不含氧化物(对于E <0.6 V vs RHE而言,是“还原的”金属态)时,接受碳表面基团(CO ad类物质),然后(ii)在众所周知的Langmuir-Hinshelwood CO-Stripping反应中将CO ad物种电氧化为CO 2,如果确实形成OH ad物种(对于E相对于RHE> 0.6 V)。结果,当在0.1 < E <0.6 V vs. RHE或0.6 < E <1.23 V vs. RHE之间进行AST时,即当Pt纳米颗粒分别被还原或氧化时,其降解过程较少强烈且不同:前者在奥斯特瓦尔德熟化过程中发生,而铂纳米颗粒在后一种情况下发生团聚。与液体电解质的情况相反,当在干电池中执行最严重的AST(0.1 < E <1.23 V vs RHE)时,降解的幅度和主要机理会发生显着变化。因为没有多余的水来溶解Pt z +由Pt纳米粒子腐蚀,3D Ostwald成熟和在现有粒子上局部再沉积形成的分子种类变得更加可能:阴离子交换离聚物更好地俘获了Pt z +并阻止其扩散到有源层之外。另外,不存在游离碱金属阳离子避免了固体碳酸盐的沉淀,因此,不利于Pt纳米颗粒从其载体上脱离。这表明给定电催化剂的降解过程不仅取决于其性质,还取决于在AST中扫描的顶点电势值,并且重要地,取决于所研究的电解质介质的性质。最后,固体碱性电解质在某种程度上减轻了Pt / C纳米粒子在液体电解质中经历的非常剧烈的降解,这为开发耐久的基于AEM的燃料电池和电解槽提供了希望。
更新日期:2018-01-18
中文翻译:

阴离子交换膜界面中Pt / C纳米粒子的加速应力测试-相同位置的透射电子显微镜研究
通过在液体碱性电解质(0.1 M NaOH)和固体阴离子交换聚合物电解质中使用“干电池”(即“干电池”)进行的加速应力测试(AST)程序,评估了最新技术的Pt / C电催化剂的耐久性。在没有液体电解质的情况下。在液体环境中,正和负顶点电势值对降解的程度和程度有很大影响:在宽电势范围内观察到的电化学表面积损失(0.1 < E相对于RHE小于1.23 V)归因于Pt纳米颗粒从其载体上脱离。Pt纳米颗粒有助于碳载体材料的局部腐蚀,最终产生固体碱金属碳酸盐,从而将其从载体上机械排出。此类腐蚀与Pt纳米颗粒倾向于(i)当碳表面基团的表面不含氧化物(对于E <0.6 V vs RHE而言,是“还原的”金属态)时,接受碳表面基团(CO ad类物质),然后(ii)在众所周知的Langmuir-Hinshelwood CO-Stripping反应中将CO ad物种电氧化为CO 2,如果确实形成OH ad物种(对于E相对于RHE> 0.6 V)。结果,当在0.1 < E <0.6 V vs. RHE或0.6 < E <1.23 V vs. RHE之间进行AST时,即当Pt纳米颗粒分别被还原或氧化时,其降解过程较少强烈且不同:前者在奥斯特瓦尔德熟化过程中发生,而铂纳米颗粒在后一种情况下发生团聚。与液体电解质的情况相反,当在干电池中执行最严重的AST(0.1 < E <1.23 V vs RHE)时,降解的幅度和主要机理会发生显着变化。因为没有多余的水来溶解Pt z +由Pt纳米粒子腐蚀,3D Ostwald成熟和在现有粒子上局部再沉积形成的分子种类变得更加可能:阴离子交换离聚物更好地俘获了Pt z +并阻止其扩散到有源层之外。另外,不存在游离碱金属阳离子避免了固体碳酸盐的沉淀,因此,不利于Pt纳米颗粒从其载体上脱离。这表明给定电催化剂的降解过程不仅取决于其性质,还取决于在AST中扫描的顶点电势值,并且重要地,取决于所研究的电解质介质的性质。最后,固体碱性电解质在某种程度上减轻了Pt / C纳米粒子在液体电解质中经历的非常剧烈的降解,这为开发耐久的基于AEM的燃料电池和电解槽提供了希望。



























 京公网安备 11010802027423号
京公网安备 11010802027423号