当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hsp70 Inhibits the Nucleation and Elongation of Tau and Sequesters Tau Aggregates with High Affinity
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acschembio.7b01039 Franziska Kundel 1 , Suman De 1 , Patrick Flagmeier 1 , Mathew H. Horrocks 1 , Magnus Kjaergaard 1 , Sarah L. Shammas 1 , Sophie E. Jackson 1 , Christopher M. Dobson 1 , David Klenerman 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acschembio.7b01039 Franziska Kundel 1 , Suman De 1 , Patrick Flagmeier 1 , Mathew H. Horrocks 1 , Magnus Kjaergaard 1 , Sarah L. Shammas 1 , Sophie E. Jackson 1 , Christopher M. Dobson 1 , David Klenerman 1
Affiliation
|
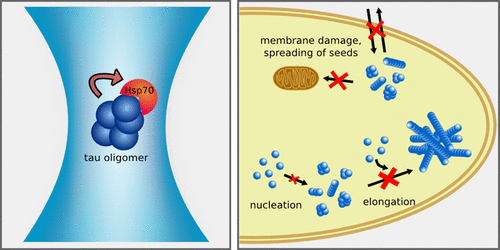
As a key player of the protein quality control network of the cell, the molecular chaperone Hsp70 inhibits the aggregation of the amyloid protein tau. To date, the mechanism of this inhibition and the tau species targeted by Hsp70 remain unknown. This is partly due to the inherent difficulty of studying amyloid aggregates because of their heterogeneous and transient nature. Here, we used ensemble and single-molecule fluorescence measurements to dissect how Hsp70 counteracts the self-assembly process of the K18 ΔK280 tau variant. We found that Hsp70 blocks the early stages of tau aggregation by suppressing the formation of tau nuclei. Additionally, Hsp70 sequesters oligomers and mature tau fibrils with nanomolar affinity into a protective complex, efficiently neutralizing their ability to damage membranes and seed further tau aggregation. Our results provide novel insights into the molecular mechanisms by which the chaperone Hsp70 counteracts the formation, propagation, and toxicity of tau aggregates.
中文翻译:

Hsp70以高亲和力抑制Tau和螯合Tau聚集体的成核和伸长
作为细胞蛋白质质量控制网络的关键参与者,分子伴侣Hsp70抑制淀粉样蛋白tau的聚集。迄今为止,这种抑制作用的机制和被Hsp70靶向的tau物种仍然未知。部分原因是由于淀粉样蛋白聚集体的异质性和短暂性,其固有的研究难度。在这里,我们使用集合和单分子荧光测量来剖析Hsp70如何抵消K18ΔK280tau变体的自组装过程。我们发现Hsp70通过抑制tau核的形成来阻止tau聚集的早期阶段。此外,Hsp70将具有纳摩尔摩尔亲和力的低聚物和成熟的tau原纤维螯合成保护性复合物,从而有效地中和其破坏膜和种子进一步tau聚集的能力。
更新日期:2018-01-04
中文翻译:

Hsp70以高亲和力抑制Tau和螯合Tau聚集体的成核和伸长
作为细胞蛋白质质量控制网络的关键参与者,分子伴侣Hsp70抑制淀粉样蛋白tau的聚集。迄今为止,这种抑制作用的机制和被Hsp70靶向的tau物种仍然未知。部分原因是由于淀粉样蛋白聚集体的异质性和短暂性,其固有的研究难度。在这里,我们使用集合和单分子荧光测量来剖析Hsp70如何抵消K18ΔK280tau变体的自组装过程。我们发现Hsp70通过抑制tau核的形成来阻止tau聚集的早期阶段。此外,Hsp70将具有纳摩尔摩尔亲和力的低聚物和成熟的tau原纤维螯合成保护性复合物,从而有效地中和其破坏膜和种子进一步tau聚集的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号