当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alamethicin self-assembling in lipid membranes: concentration dependence from pulsed EPR of spin labels†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7cp07298h Victoria N. Syryamina 1, 2, 3, 4, 5 , Marta De Zotti 6, 7, 8, 9 , Claudio Toniolo 6, 7, 8, 9, 10 , Fernando Formaggio 6, 7, 8, 9, 10 , Sergei A. Dzuba 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7cp07298h Victoria N. Syryamina 1, 2, 3, 4, 5 , Marta De Zotti 6, 7, 8, 9 , Claudio Toniolo 6, 7, 8, 9, 10 , Fernando Formaggio 6, 7, 8, 9, 10 , Sergei A. Dzuba 1, 2, 3, 4, 5
Affiliation
|
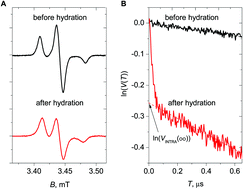
The antimicrobial action of the peptide antibiotic alamethicin (Alm) is commonly related to peptide self-assembling resulting in the formation of voltage-dependent channels in bacterial membranes, which induces ion permeation. To obtain a deeper insight into the mechanism of channel formation, it is useful to know the dependence of self-assembling on peptide concentration. With this aim, we studied Alm F50/5 spin-labeled analogs in a model 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) membrane, for peptide-to-lipid (P/L) ratios varying between 1/1500 and 1/100. Pulsed electron–electron double resonance (PELDOR) spectroscopy reveals that even at the lowest concentration investigated, the Alm molecules assemble into dimers. Moreover, under these conditions, electron spin echo envelope modulation (ESEEM) spectroscopy of D2O-hydrated membranes shows an abrupt change from the in-plane to the trans-membrane orientation of the peptide. Therefore, we hypothesize that dimer formation and peptide reorientation are concurrent processes and represent the initial step of peptide self-assembling. By increasing peptide concentration, higher oligomers are formed. A simple kinetic model of equilibrium among monomers, dimers, and pentamers allows for satisfactorily describing the experimental PELDOR data. The inter-label distances in the oligomers obtained from PELDOR experiments become better resolved with increasing P/L ratio, thus suggesting that the supramolecular organization of the higher-order oligomers becomes more defined.
中文翻译:

脂膜中的Alamethicin自组装:自旋标记物的脉冲EPR的浓度依赖性†
肽抗菌素阿乐霉素(Alm)的抗菌作用通常与肽自组装有关,导致细菌膜中电压依赖性通道的形成,从而引起离子渗透。为了更深入地了解通道形成的机制,了解自组装对肽浓度的依赖性非常有用。为此,我们在1-棕榈酰-2-油酰-sn-甘油模型中研究了Alm F50 / 5自旋标记的类似物-3-磷酸胆碱(POPC)膜,肽与脂质(P / L)的比率在1/1500和1/100之间变化。脉冲电子-电子双共振(PELDOR)光谱显示,即使以最低的研究浓度,Alm分子也会组装成二聚体。此外,在这些条件下,D 2的电子自旋回波包络调制(ESEEM)光谱O-水合膜显示出从肽的平面内到跨膜方向的突然变化。因此,我们假设二聚体的形成和肽的重新定向是同时发生的过程,代表了肽自组装的起始步骤。通过增加肽浓度,形成更高的低聚物。单体,二聚体和五聚体之间平衡的简单动力学模型可以令人满意地描述实验性PELDOR数据。从PELDOR实验获得的低聚物中的标记间距离随着P / L比的增加而得到更好的解析,因此表明高阶低聚物的超分子组织变得更加明确。
更新日期:2018-01-05
中文翻译:

脂膜中的Alamethicin自组装:自旋标记物的脉冲EPR的浓度依赖性†
肽抗菌素阿乐霉素(Alm)的抗菌作用通常与肽自组装有关,导致细菌膜中电压依赖性通道的形成,从而引起离子渗透。为了更深入地了解通道形成的机制,了解自组装对肽浓度的依赖性非常有用。为此,我们在1-棕榈酰-2-油酰-sn-甘油模型中研究了Alm F50 / 5自旋标记的类似物-3-磷酸胆碱(POPC)膜,肽与脂质(P / L)的比率在1/1500和1/100之间变化。脉冲电子-电子双共振(PELDOR)光谱显示,即使以最低的研究浓度,Alm分子也会组装成二聚体。此外,在这些条件下,D 2的电子自旋回波包络调制(ESEEM)光谱O-水合膜显示出从肽的平面内到跨膜方向的突然变化。因此,我们假设二聚体的形成和肽的重新定向是同时发生的过程,代表了肽自组装的起始步骤。通过增加肽浓度,形成更高的低聚物。单体,二聚体和五聚体之间平衡的简单动力学模型可以令人满意地描述实验性PELDOR数据。从PELDOR实验获得的低聚物中的标记间距离随着P / L比的增加而得到更好的解析,因此表明高阶低聚物的超分子组织变得更加明确。











































 京公网安备 11010802027423号
京公网安备 11010802027423号