Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modified and ion exchanged clinoptilolite for the adsorptive removal of sulfur compounds in a model fuel: New adsorbents for desulfurization
Fuel ( IF 7.4 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.fuel.2017.12.095 Milad Moradi , Ramin Karimzadeh , Elham Sadat Moosavi
Fuel ( IF 7.4 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.fuel.2017.12.095 Milad Moradi , Ramin Karimzadeh , Elham Sadat Moosavi
|
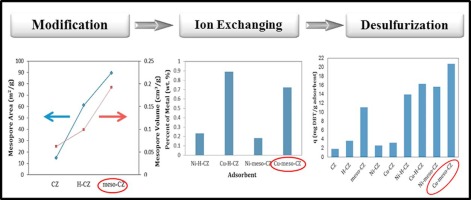
Abstract In this research, modified and ion exchanged natural clinoptilolite (CZ) were prepared for adsorptive desulfurization of refractory dibenzothiophene (DBT) and 4,6-dimethyl dibenzothiophene (4,6-DMDBT) sulfur compounds in a model fuel. For this purpose, H-form (H-CZ) and mesopore (meso-CZ) clinoptilolite were prepared and then ion exchanged with Cu+ and Ni2+ cations. The raw and treated samples were characterized by X-ray diffraction (XRD), X-ray fluorescence (XRF), FTIR spectroscopy and N2 adsorption-desorption (BET) analysis. The obtained surface areas of CZ, H-CZ and meso-CZ were 19.6, 249.71 and 194.65 m2/g, and their obtained total pore volumes were 0.0644, 0.1869 and 0.2404 cm3/g, respectively. In Ni2+- and Cu+-exchanged adsorbents due to presence of d-electrons, sulfur compounds interact with the cations through π-complexation which resulted in significant increase adsorption capacity of ion exchanged samples, compared to parent samples. The experimental data were analyzed using Langmuir, Freundlich, Langmuir-Freundlich (L-F) and Dubinin-Radushkevich (D-R) isotherms. For adsorption of DBT onto all adsorbents, L-F was the best-fitting isotherm to the experimental data. Due to high surface area and mesopore volume, large pore width, higher ion exchange compared to Ni-meso-CZ, and also formation of π-complexation between sulfur atom and Cu+ cation, Cu-meso-CZ gives the best results for removal of DBT (28.12 mg DBT/g adsorbent) based on L-F isotherm. In addition, the effect of solvent and sulfur compound on adsorption capacity of adsorbents and regeneration of them were investigated by thermal method.
中文翻译:

用于吸附去除模型燃料中硫化合物的改性和离子交换斜发沸石:用于脱硫的新型吸附剂
摘要 本研究制备了改性离子交换天然斜发沸石(CZ),用于模型燃料中难熔二苯并噻吩(DBT)和4,6-二甲基二苯并噻吩(4,6-DMDBT)硫化合物的吸附脱硫。为此,制备了 H 型 (H-CZ) 和介孔 (meso-CZ) 斜发沸石,然后与 Cu+ 和 Ni2+ 阳离子进行离子交换。通过 X 射线衍射 (XRD)、X 射线荧光 (XRF)、FTIR 光谱和 N2 吸附-解吸 (BET) 分析对原始样品和处理过的样品进行表征。得到的CZ、H-CZ和meso-CZ的表面积分别为19.6、249.71和194.65 m2/g,获得的总孔体积分别为0.0644、0.1869和0.2404 cm3/g。由于 d 电子的存在,在 Ni2+- 和 Cu+- 交换的吸附剂中,与母体样品相比,硫化合物通过 π 络合与阳离子相互作用,导致离子交换样品的吸附能力显着增加。使用 Langmuir、Freundlich、Langmuir-Freundlich (LF) 和 Dubinin-Radushkevich (DR) 等温线分析实验数据。对于 DBT 在所有吸附剂上的吸附,LF 是实验数据的最佳拟合等温线。由于比 Ni-meso-CZ 具有高表面积和中孔体积、大孔宽、更高的离子交换率以及硫原子和 Cu+ 阳离子之间形成 π 络合物,Cu-meso-CZ 提供了最佳的去除效果DBT(28.12 mg DBT/g 吸附剂)基于 LF 等温线。此外,通过热法研究了溶剂和硫化合物对吸附剂吸附能力和再生的影响。
更新日期:2018-04-01
中文翻译:

用于吸附去除模型燃料中硫化合物的改性和离子交换斜发沸石:用于脱硫的新型吸附剂
摘要 本研究制备了改性离子交换天然斜发沸石(CZ),用于模型燃料中难熔二苯并噻吩(DBT)和4,6-二甲基二苯并噻吩(4,6-DMDBT)硫化合物的吸附脱硫。为此,制备了 H 型 (H-CZ) 和介孔 (meso-CZ) 斜发沸石,然后与 Cu+ 和 Ni2+ 阳离子进行离子交换。通过 X 射线衍射 (XRD)、X 射线荧光 (XRF)、FTIR 光谱和 N2 吸附-解吸 (BET) 分析对原始样品和处理过的样品进行表征。得到的CZ、H-CZ和meso-CZ的表面积分别为19.6、249.71和194.65 m2/g,获得的总孔体积分别为0.0644、0.1869和0.2404 cm3/g。由于 d 电子的存在,在 Ni2+- 和 Cu+- 交换的吸附剂中,与母体样品相比,硫化合物通过 π 络合与阳离子相互作用,导致离子交换样品的吸附能力显着增加。使用 Langmuir、Freundlich、Langmuir-Freundlich (LF) 和 Dubinin-Radushkevich (DR) 等温线分析实验数据。对于 DBT 在所有吸附剂上的吸附,LF 是实验数据的最佳拟合等温线。由于比 Ni-meso-CZ 具有高表面积和中孔体积、大孔宽、更高的离子交换率以及硫原子和 Cu+ 阳离子之间形成 π 络合物,Cu-meso-CZ 提供了最佳的去除效果DBT(28.12 mg DBT/g 吸附剂)基于 LF 等温线。此外,通过热法研究了溶剂和硫化合物对吸附剂吸附能力和再生的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号