Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.tetlet.2018.01.006 Murray G. Rosenberg , Udo H. Brinker
|
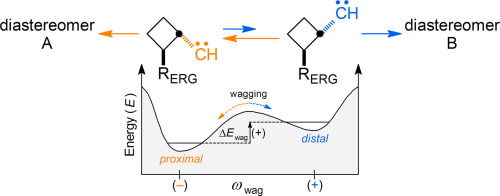
The UM06-2X/aug-cc-pVTZ//UM06-2X/6-311G(d) theoretical models of representative singlet trans-2′-substituted cyclobutylcarbenes were computed. The :CH-group of carbenes with an ERG is bent toward the c-C4H7-ring’s proximal C1′–C2′ single bond. The :CH-group of carbenes with an EWG is bent toward the c-C4H7-ring’s distal C1′–C4′ single bond. The contrasting stereoelectronic effect was computationally tested using trimethylsilyl vs. bromo substituents to determine if exo-1- and exo-2-substituted bicyclo[2.1.0]pentanes would be produced in diastereomeric excess.
中文翻译:

非对映选择性碳烯:弯曲单线态反式-2'-取代的环丁基卡宾的立体电子控制
计算了代表性单重态反式-2'-取代的环丁基卡宾的UM06-2X / aug-cc-pVTZ // UM06-2X / 6-311G(d)理论模型。的: CH-基与ERG卡宾是向弯曲Ç -C 4 ħ 7形环的近侧C1'-C2'单键。的: CH-组以EWG卡宾是向弯曲Ç -C 4 ħ 7形环的远侧C1'-C4'单键。使用三甲基甲硅烷基与溴取代基计算对比了立体电子效应,以确定exo -1-和exo-2-取代的双环[2.1.0]戊烷将以非对映异构体过量的形式产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号