当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights on Graphite Anode Stability in Rechargeable Batteries: Li Ion Coordination Structures Prevail over Solid Electrolyte Interphases
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acsenergylett.7b01177 Jun Ming 1 , Zhen Cao 1 , Wandi Wahyudi 1 , Mengliu Li 1 , Pushpendra Kumar 1 , Yingqiang Wu 1 , Jang-Yeon Hwang 2 , Mohamed Nejib Hedhili 1 , Luigi Cavallo 1 , Yang-Kook Sun 2 , Lain-Jong Li 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acsenergylett.7b01177 Jun Ming 1 , Zhen Cao 1 , Wandi Wahyudi 1 , Mengliu Li 1 , Pushpendra Kumar 1 , Yingqiang Wu 1 , Jang-Yeon Hwang 2 , Mohamed Nejib Hedhili 1 , Luigi Cavallo 1 , Yang-Kook Sun 2 , Lain-Jong Li 1
Affiliation
|
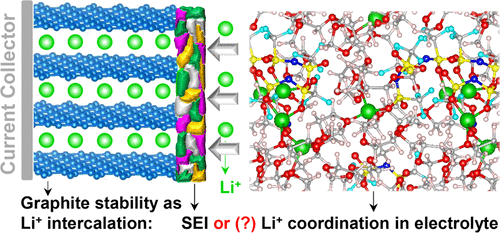
Graphite anodes are not stable in most noncarbonate solvents (e.g., ether, sulfoxide, sulfone) upon Li ion intercalation, known as an urgent issue in present Li ions and next-generation Li–S and Li–O2 batteries for storage of Li ions within the anode for safety features. The solid electrolyte interphase (SEI) is commonly believed to be decisive for stabilizing the graphite anode. However, here we find that the solvation structure of the Li ions, determined by the electrolyte composition including lithium salts, solvents, and additives, plays a more dominant role than SEI in graphite anode stability. The Li ion intercalation desired for battery operation competes with the undesired Li+–solvent co-insertion, leading to graphite exfoliation. The increase in organic lithium salt LiN(SO2CF3)2 concentration or, more effectively, the addition of LiNO3 lowers the interaction strength between Li+ and solvents, suppressing the graphite exfoliation caused by Li+–solvent co-insertion. Our findings refresh the knowledge of the well-known SEI for graphite stability in metal ion batteries and also provide new guidelines for electrolyte systems to achieve reliable and safe Li–S full batteries.
中文翻译:

充电电池中石墨阳极稳定性的新见解:锂离子配位结构优于固体电解质中间相
嵌入锂离子后,石墨阳极在大多数非碳酸盐溶剂(例如,乙醚,亚砜,砜)中不稳定,这在当前的锂离子以及用于存储锂离子的下一代Li–S和Li–O 2电池中是一个紧迫的问题在阳极内以确保安全性。通常认为固体电解质相(SEI)对于稳定石墨阳极起决定性作用。但是,在这里我们发现,由包括锂盐,溶剂和添加剂的电解质组成决定的Li离子的溶剂化结构在石墨阳极稳定性方面比SEI更具主导作用。电池运行所需的锂离子嵌入层与不希望的Li +-溶剂共嵌入层竞争,从而导致石墨剥落。有机锂盐LiN(SO2 CF 3)2浓度,或更有效地,添加LiNO 3降低了Li +与溶剂之间的相互作用强度,从而抑制了由Li +-溶剂共插入引起的石墨剥落。我们的发现刷新了众所周知的SEI在金属离子电池中石墨稳定性方面的知识,并且还为电解质系统提供了新的指南,以实现可靠和安全的Li–S充满电池。
更新日期:2018-01-10
中文翻译:

充电电池中石墨阳极稳定性的新见解:锂离子配位结构优于固体电解质中间相
嵌入锂离子后,石墨阳极在大多数非碳酸盐溶剂(例如,乙醚,亚砜,砜)中不稳定,这在当前的锂离子以及用于存储锂离子的下一代Li–S和Li–O 2电池中是一个紧迫的问题在阳极内以确保安全性。通常认为固体电解质相(SEI)对于稳定石墨阳极起决定性作用。但是,在这里我们发现,由包括锂盐,溶剂和添加剂的电解质组成决定的Li离子的溶剂化结构在石墨阳极稳定性方面比SEI更具主导作用。电池运行所需的锂离子嵌入层与不希望的Li +-溶剂共嵌入层竞争,从而导致石墨剥落。有机锂盐LiN(SO2 CF 3)2浓度,或更有效地,添加LiNO 3降低了Li +与溶剂之间的相互作用强度,从而抑制了由Li +-溶剂共插入引起的石墨剥落。我们的发现刷新了众所周知的SEI在金属离子电池中石墨稳定性方面的知识,并且还为电解质系统提供了新的指南,以实现可靠和安全的Li–S充满电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号