Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.mcat.2017.12.036 Céline Tisseraud , Clément Comminges , Aurélien Habrioux , Stéphane Pronier , Yannick Pouilloux , Anthony Le Valant
|
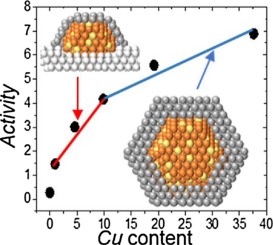
Synthesis of Cu-ZnO catalysts for the direct hydrogenation of CO2 to methanol requires catalyst precursors able to display a strong Cu-Zn interaction. Copper and zinc mixed hydroxynitrates are good candidates to fulfill this specification. The synthesis of such a phase was achieved by a wet impregnation of copper nitrate on ZnO. Solvent evaporation and catalyst drying at moderate temperatures allowed retaining the metals hydroxynitrate structure, leading to a memory effect for the reduced catalysts. Depending on the Cu content in the reaction mixture, a ZnO lixiviation gradually occurs, what induces an important change in the morphology of the reduced catalyst. Catalyst activity is proportional to the amount of Zn migrated in the active phase, the latter consisting most probably of an oxygen deficient mixed oxide comprising both Cu and Zn. The most active catalyst is obtained when ZnO is fully lixiviated during the catalyst precursor synthesis, giving a core-shell structure after reduction.
中文翻译:

用于CO 2加氢制甲醇的Cu-ZnO催化剂:ZnO浸沉诱导的形貌变化及其对活性相形成的影响
用于CO 2直接加氢的Cu-ZnO催化剂的合成甲醇制甲醇需要催化剂前体能够显示出强大的Cu-Zn相互作用。铜和锌的混合硝酸硝酸盐是满足该规格的良好选择。这种相的合成是通过将硝酸铜湿法浸渍在ZnO上来实现的。溶剂蒸发和催化剂在适中的温度下干燥可以保留金属羟基硝酸盐的结构,从而对减少的催化剂产生记忆效应。取决于反应混合物中的Cu含量,逐渐发生ZnO浸出,这引起了还原催化剂形态的重要变化。催化剂活性与活性相中迁移的Zn量成正比,后者很可能由同时含有Cu和Zn的缺氧混合氧化物组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号