当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Library-to-Library Synthesis of Highly Substituted α-Aminomethyl Tetrazoles via Ugi Reaction.
ACS Combinatorial Science Pub Date : 2018-01-04 , DOI: 10.1021/acscombsci.7b00137 Pravin Patil 1 , Bhupendra Mishra 1 , Gitanjali Sheombarsing 1 , Katarzyna Kurpiewska 2 , Justyna Kalinowska-Tłuścik 2 , Alexander Dömling 1
ACS Combinatorial Science Pub Date : 2018-01-04 , DOI: 10.1021/acscombsci.7b00137 Pravin Patil 1 , Bhupendra Mishra 1 , Gitanjali Sheombarsing 1 , Katarzyna Kurpiewska 2 , Justyna Kalinowska-Tłuścik 2 , Alexander Dömling 1
Affiliation
|
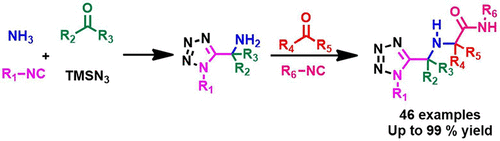
α-Aminomethyl tetrazoles, recently made accessible by an Ugi multicomponent reaction (MCR), were shown to be excellent starting materials for a further Ugi MCR, yielding substituted N-methyl-2-(((1-methyl-1H-tetrazol-5-yl)methyl)amino)acetamides having four points of diversity in a library-to-library approach. The scope and limitations of the two-step sequence was explored by conducting more than 50 reactions. Irrespective of electron-rich and electron-deficient oxo-components and the nature of the isocyanide component, the reactions give excellent yields. Sterically less hindered α-aminomethyl tetrazoles give better yields of in further Ugi MCR. The target scaffold has four points of diversity and is finding applications to fill screening decks for high-throughput screening (HTS) in the European Lead Factory and in structure-based drug design.
中文翻译:

通过 Ugi 反应进行高度取代的 α-氨基甲基四唑的文库合成。
最近通过 Ugi 多组分反应 (MCR) 获得的 α-氨基甲基四唑被证明是进一步 Ugi MCR 的优异起始材料,产生取代的 N-甲基-2-(((1-甲基-1H-四唑-5) -基)甲基)氨基)乙酰胺在文库间方法中具有四个多样性点。通过进行 50 多个反应,探索了两步序列的范围和局限性。无论富电子和缺电子含氧组分以及异氰化物组分的性质如何,该反应都具有优异的产率。空间位阻较小的 α-氨甲基四唑可进一步提高 Ugi MCR 的产率。目标支架具有四个多样性点,正在寻找填充欧洲先导工厂高通量筛选 (HTS) 筛选平台和基于结构的药物设计的应用。
更新日期:2018-01-04
中文翻译:

通过 Ugi 反应进行高度取代的 α-氨基甲基四唑的文库合成。
最近通过 Ugi 多组分反应 (MCR) 获得的 α-氨基甲基四唑被证明是进一步 Ugi MCR 的优异起始材料,产生取代的 N-甲基-2-(((1-甲基-1H-四唑-5) -基)甲基)氨基)乙酰胺在文库间方法中具有四个多样性点。通过进行 50 多个反应,探索了两步序列的范围和局限性。无论富电子和缺电子含氧组分以及异氰化物组分的性质如何,该反应都具有优异的产率。空间位阻较小的 α-氨甲基四唑可进一步提高 Ugi MCR 的产率。目标支架具有四个多样性点,正在寻找填充欧洲先导工厂高通量筛选 (HTS) 筛选平台和基于结构的药物设计的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号