当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isoenergic modification of whey protein structure by denaturation and crosslinking using transglutaminase†
Food & Function ( IF 5.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7fo01451a Emil G. P. Stender 1, 2, 3 , Glykeria Koutina 3, 4, 5, 6 , Kristoffer Almdal 2, 3, 7 , Tue Hassenkam 3, 5, 6, 8 , Alan Mackie 9, 10, 11, 12, 13 , Richard Ipsen 3, 4, 5, 6 , Birte Svensson 1, 2, 3
Food & Function ( IF 5.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7fo01451a Emil G. P. Stender 1, 2, 3 , Glykeria Koutina 3, 4, 5, 6 , Kristoffer Almdal 2, 3, 7 , Tue Hassenkam 3, 5, 6, 8 , Alan Mackie 9, 10, 11, 12, 13 , Richard Ipsen 3, 4, 5, 6 , Birte Svensson 1, 2, 3
Affiliation
|
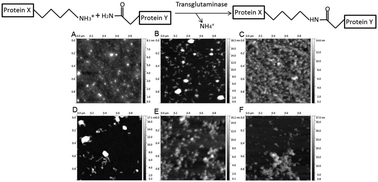
Transglutaminase (TG) catalyzes formation of covalent bonds between lysine and glutamine side chains and has applications in manipulation of food structure. Physical properties of a whey protein mixture (SPC) denatured either at elevated pH or by heat-treatment and followed by TG catalyzed crosslinking, have been characterised using dynamic light scattering, size exclusion chromatography, flourescence spectroscopy and atomic force microscopy. The degree of enzymatic crosslinking appeared higher for pH- than for heat-denatured SPC. The hydrophobic surface properties depended on the treatment, thus heating caused the largest exposure of the hydrophobic core of SPC proteins, which was decreased by crosslinking. The particle size of the treated SPC samples increased upon crosslinking by TG. Moreover, the particle morphology depended on the type of denaturing treatment, thus heat-treated SPC contained fibrillar structures, while pH-denatured SPC remained globular as documented by using atomic force microscopy. Finally, the in vitro digestability of the different SPC samples was assessed under simulated gastric and intestinal conditions. Notably heat-treatment was found to lower the gastric digestion rate and enzymatic crosslinking reduced both the gastric and the intestinal rate of digestion. These characteristics of the various SPC samples provide a useful basis for design of isoenergic model foods applicable in animal and human studies on how food structure affects satiety.
中文翻译:

通过变性乳清蛋白结构的等能修饰和使用转谷氨酰胺酶交联†
转谷氨酰胺酶(TG)催化赖氨酸和谷氨酰胺侧链之间共价键的形成,并在控制食物结构中有应用。使用动态光散射,尺寸排阻色谱,荧光光谱和原子力显微镜对表征的乳清蛋白混合物(SPC)的物理性质进行了表征,该混合物在升高的pH值下或通过热处理而变性,然后通过TG催化交联。pH值下的酶交联度似乎比热变性SPC的高。疏水性表面性质取决于处理,因此加热导致SPC蛋白疏水性核心的最大暴露,而交联降低了SPC蛋白的疏水性核心。经TG交联后,处理过的SPC样品的粒度增加。而且,粒子的形态取决于变性处理的类型,因此热处理的SPC含有原纤维结构,而pH改性的SPC仍呈球状,如使用原子力显微镜所证明的那样。最后,在模拟的胃和肠条件下评估了不同SPC样品的体外消化率。显着地发现热处理降低了胃的消化速率,并且酶促交联降低了胃和肠的消化速率。各种SPC样品的这些特征为设计等能量模型食品提供了有用的基础,这些模型可用于动物和人类对食物结构如何影响饱腹感的研究。
更新日期:2018-01-05
中文翻译:

通过变性乳清蛋白结构的等能修饰和使用转谷氨酰胺酶交联†
转谷氨酰胺酶(TG)催化赖氨酸和谷氨酰胺侧链之间共价键的形成,并在控制食物结构中有应用。使用动态光散射,尺寸排阻色谱,荧光光谱和原子力显微镜对表征的乳清蛋白混合物(SPC)的物理性质进行了表征,该混合物在升高的pH值下或通过热处理而变性,然后通过TG催化交联。pH值下的酶交联度似乎比热变性SPC的高。疏水性表面性质取决于处理,因此加热导致SPC蛋白疏水性核心的最大暴露,而交联降低了SPC蛋白的疏水性核心。经TG交联后,处理过的SPC样品的粒度增加。而且,粒子的形态取决于变性处理的类型,因此热处理的SPC含有原纤维结构,而pH改性的SPC仍呈球状,如使用原子力显微镜所证明的那样。最后,在模拟的胃和肠条件下评估了不同SPC样品的体外消化率。显着地发现热处理降低了胃的消化速率,并且酶促交联降低了胃和肠的消化速率。各种SPC样品的这些特征为设计等能量模型食品提供了有用的基础,这些模型可用于动物和人类对食物结构如何影响饱腹感的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号