当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What Contributes to Serotonin–Norepinephrine Reuptake Inhibitors’ Dual-Targeting Mechanism? The Key Role of Transmembrane Domain 6 in Human Serotonin and Norepinephrine Transporters Revealed by Molecular Dynamics Simulation
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acschemneuro.7b00490 Weiwei Xue 1, 2 , Fengyuan Yang 1, 2 , Panpan Wang 1, 2 , Guoxun Zheng 1, 2 , Yuzong Chen 3 , Xiaojun Yao 4 , Feng Zhu 1, 2
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acschemneuro.7b00490 Weiwei Xue 1, 2 , Fengyuan Yang 1, 2 , Panpan Wang 1, 2 , Guoxun Zheng 1, 2 , Yuzong Chen 3 , Xiaojun Yao 4 , Feng Zhu 1, 2
Affiliation
|
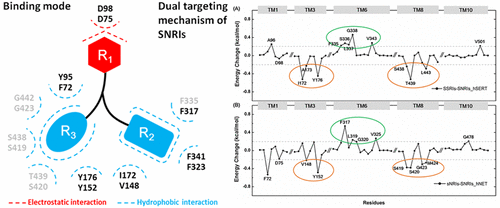
Dual inhibition of serotonin and norepinephrine transporters (hSERT and hNET) gives greatly improved efficacy and tolerability for treating major depressive disorder (MDD) compared with selective reuptake inhibitors. Pioneer studies provided valuable information on structure, function, and pharmacology of drugs targeting both hSERT and hNET (serotonin–norepinephrine reuptake inhibitors, SNRIs), and the differential binding mechanism between SNRIs and selective inhibitors of 5-HT (SSRIs) or NE (sNRIs) to their corresponding targets was expected to be able to facilitate the discovery of a privileged drug-like scaffold with improved efficacy. However, the dual-target mechanism of SNRIs was still elusive, and the binding mode distinguishing SNRIs from SSRIs and sNRIs was also unclear. Herein, an integrated computational strategy was adopted to discover the binding mode shared by all FDA approved SNRIs. The comparative analysis of binding free energy at the per-residue level discovered that residues Phe335, Leu337, Gly338, and Val343 located at the transmembrane domain 6 (TM6) of hSERT (the corresponding residues Phe317, Leu319, Gly320, and Val325 in hNET) were the determinants accounting for SNRIs’ dual-acting inhibition, while residues lining TM3 and 8 (Ile172, Ser438, Thr439, and Leu443 in hSERT; Val148, Ser419, Ser420, and Met424 in hNET) contributed less to the binding of SNRIs than that of SSRIs and sNRIs. Based on these results, the distances between an SNRI’s centroid and the centroids of its two aromatic rings (measuring the depth of rings stretching into hydrophobic pockets) were discovered as the key to the SNRIs’ dual-targeting mechanism. This finding revealed SNRIs’ binding mechanism at an atomistic level, which could be further utilized as structural blueprints for the rational design of privileged drug-like scaffolds treating MDD.
中文翻译:

什么原因导致5-羟色胺-去甲肾上腺素再摄取抑制剂的双重靶向机制?分子动力学模拟揭示跨膜结构域6在人血清素和去甲肾上腺素转运蛋白中的关键作用
与选择性再摄取抑制剂相比,对5-羟色胺和去甲肾上腺素转运蛋白(hSERT和hNET)的双重抑制大大改善了治疗重度抑郁症(MDD)的功效和耐受性。先锋研究提供了有关靶向hSERT和hNET(血清素-去甲肾上腺素再摄取抑制剂,SNRI)的药物的结构,功能和药理学的有价值信息,以及SNRI与5-HT(SSRIs)或NE(sNRIs)选择性抑制剂之间的差异结合机制。 )有望达到其相应目标,从而能够促进发现具有改善功效的特异药物样支架。但是,SNRI的双目标机制仍然难以捉摸,并且区分SNRI与SSRI和sNRI的结合模式也不清楚。在此处,采用了一种集成的计算策略来发现所有FDA批准的SNRI共享的结合模式。在每个残基水平的结合自由能的比较分析发现,残基Phe335,Leu337,Gly338和Val343位于hSERT的跨膜结构域6(TM6)(hNET中的相应残基Phe317,Leu319,Gly320和Val325)是解释SNRIs双重作用抑制的决定因素,而TM3和8(hSERT中的Ile172,Ser438,Thr439和Leu443; hNET中的Val148,Ser419,Ser420和Met424)内衬的残基对SNRIs的结合的贡献小于SSRI和sNRI。根据这些结果,发现SNRI质心与其两个芳香环的质心之间的距离(测量伸入疏水性口袋的环的深度)是SNRIs双靶向机制的关键。这一发现揭示了SNRIs在原子水平上的结合机制,该机制可进一步用作结构设计蓝图,以合理设计治疗MDD的特异药物样支架。
更新日期:2018-01-04
中文翻译:

什么原因导致5-羟色胺-去甲肾上腺素再摄取抑制剂的双重靶向机制?分子动力学模拟揭示跨膜结构域6在人血清素和去甲肾上腺素转运蛋白中的关键作用
与选择性再摄取抑制剂相比,对5-羟色胺和去甲肾上腺素转运蛋白(hSERT和hNET)的双重抑制大大改善了治疗重度抑郁症(MDD)的功效和耐受性。先锋研究提供了有关靶向hSERT和hNET(血清素-去甲肾上腺素再摄取抑制剂,SNRI)的药物的结构,功能和药理学的有价值信息,以及SNRI与5-HT(SSRIs)或NE(sNRIs)选择性抑制剂之间的差异结合机制。 )有望达到其相应目标,从而能够促进发现具有改善功效的特异药物样支架。但是,SNRI的双目标机制仍然难以捉摸,并且区分SNRI与SSRI和sNRI的结合模式也不清楚。在此处,采用了一种集成的计算策略来发现所有FDA批准的SNRI共享的结合模式。在每个残基水平的结合自由能的比较分析发现,残基Phe335,Leu337,Gly338和Val343位于hSERT的跨膜结构域6(TM6)(hNET中的相应残基Phe317,Leu319,Gly320和Val325)是解释SNRIs双重作用抑制的决定因素,而TM3和8(hSERT中的Ile172,Ser438,Thr439和Leu443; hNET中的Val148,Ser419,Ser420和Met424)内衬的残基对SNRIs的结合的贡献小于SSRI和sNRI。根据这些结果,发现SNRI质心与其两个芳香环的质心之间的距离(测量伸入疏水性口袋的环的深度)是SNRIs双靶向机制的关键。这一发现揭示了SNRIs在原子水平上的结合机制,该机制可进一步用作结构设计蓝图,以合理设计治疗MDD的特异药物样支架。










































 京公网安备 11010802027423号
京公网安备 11010802027423号