Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.bioorg.2017.12.036 Fujun Dai , Haoying He , Xiaojuan Xu , Shuai Chen , Chaojie Wang , Chenyang Feng , Zhiyong Tian , Huanyang Dong , Songqiang Xie
|
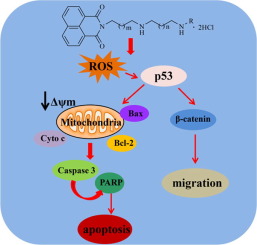
In this study, a series of novel naphthalimide-polyamine conjugates modified by alkylation at the terminal of the polyamine chain were synthesized. These novel conjugates were evaluated for their anti-cancer activities. The results revealed that the length of the polyamine chain and the terminal alkyl group had influences on anticancer activities. Compound 3g was chosen to further study the anti-cancer mechanism and evaluate the anti-tumor efficacy in vivo. It induced intrinsic apoptosis and suppressed migration of hepatoma cells. The preliminary studies of compound 3g in vivo showed that it might be a promising candidate for cancer therapy.
中文翻译:

烷基化修饰的萘二甲酰亚胺-多胺共轭物通过p53途径的合成及生物学评价
在这项研究中,合成了一系列通过在多胺链末端进行烷基化修饰的新型萘二甲酰亚胺-多胺共轭物。对这些新型缀合物的抗癌活性进行了评估。结果表明,多胺链的长度和末端烷基对抗癌活性有影响。选择化合物3g以进一步研究其抗癌机理并评估其体内抗肿瘤功效。它诱导内在凋亡并抑制肝癌细胞的迁移。体内3g 化合物的初步研究表明,它可能是有希望的癌症治疗候选药物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号