Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.bioorg.2018.01.006 Preeti Chanalia , Dimpi Gandhi , Pooja Attri , Suman Dhanda
|
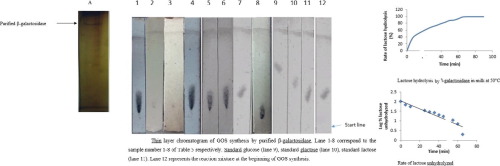
β-galactosidase is a commercially important enzyme that was purified from probiotic Pediococcus acidilactici. The enzyme was extracted from cells using sonication and subsequently purified using ammonium sulphate fractionation and successive chromatographies on Sephadex G-100 and Q-Sepharose. The enzyme was purified 3.06-fold up to electrophoretic homogeneity with specific activity of 0.883 U/mg and yield of 28.26%. Molecular mass of β-galactosidase as estimated by SDS-PAGE and MALDI-TOF was 39.07 kDa. The enzyme is a heterodimer with subunit mass of 15.55 and 19.58 kDa. The purified enzyme was optimally active at pH 6.0 and stable in a pH range of 5.8–7.0 with more than 97% activity. Purified β-galactosidase was optimally active at 50 °C. Kinetic parameters Km and Vmax for purified enzyme were 400 µM and 1.22 × 10−1 U respectively. Its inactivation by PMSF confirmed the presence of serine at the active site. The metal ions had different effects on enzyme. Ca2+, Mg2+ and Mn2+ slightly activated the enzyme whereas NH4+, Co2+ and Fe3+ slightly decreased the enzyme activity. Thermodynamic parameters were calculated that suggested that β-galactosidase is less stable at higher temperature (60 °C). Purified enzyme effectively hydrolysed milk lactose with lactose hydrolysing rate of 0.047 min−1 and t1/2 of 14.74 min. This is better than other studied β-galactosidases. Both sonicated Pediococcus acidilactici cells and purified β-galactosidase synthesized galactooligosaccharides (GOSs) as studied by TLC at 30% and 50% of lactose concentration at 47.5 °C. These findings indicate the use of β-galactosidase from probiotic bacteria for producing delactosed milk for lactose intolerant population and prebiotic synthesis. pH and temperature optima and its activation by Ca2+ shows that it is suitable for milk processing.
中文翻译:

益生菌乳酸双球菌β-半乳糖苷酶的纯化,鉴定及其在牛奶乳糖水解和半乳糖低聚糖合成中的应用
β-半乳糖苷酶是一种商业上重要的酶,是从益生菌乳酸双球菌中纯化得到的。使用超声从细胞中提取酶,然后使用硫酸铵分级分离和连续色谱法在Sephadex G-100和Q-Sepharose上纯化。将该酶纯化3.06倍至电泳均质,比活性为0.883U / mg,产率为28.26%。通过SDS-PAGE和MALDI-TOF估计的β-半乳糖苷酶的分子量为39.07 kDa。该酶是具有15.55和19.58kDa的亚基质量的异二聚体。纯化的酶在pH 6.0时具有最佳活性,在5.8-7.0的pH范围内稳定,活性超过97%。纯化的β-半乳糖苷酶在50°C时具有最佳活性。动力学参数K m和V max纯化的酶的分别为400μM和1.22×10 -1U。PMSF将其灭活,证实了在活性位点存在丝氨酸。金属离子对酶的影响不同。Ca 2 +,Mg 2+和Mn 2+轻微激活了酶,而NH 4 +,Co 2+和Fe 3+则稍微降低了酶活性。计算得出的热力学参数表明,β-半乳糖苷酶在较高温度(60°C)下不稳定。纯化的酶可有效水解乳乳糖,乳糖的水解速率为0.047 min -1和t 1/214.74分钟。这比其他研究过的β-半乳糖苷酶要好。经TLC研究,在47.5°C的30%和50%的乳糖浓度下,超声处理的乳酸双球菌乳酸细胞和纯化的β-半乳糖苷酶合成的半乳糖寡糖(GOS)。这些发现表明,将益生菌中的β-半乳糖苷酶用于生产乳糖不耐症人群和益生元合成的脱乳糖牛奶。最适的pH和温度及其被Ca 2+活化表明它适用于牛奶加工。









































 京公网安备 11010802027423号
京公网安备 11010802027423号