Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.bioorg.2018.01.004 Mietha M. van der Walt , Gisella Terre'Blanche
|
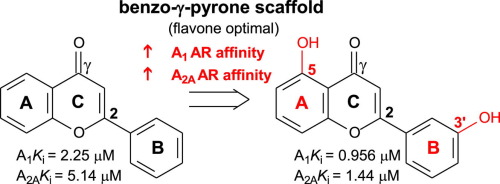
Adenosine receptor antagonists are under investigation as potential drug candidates for the treatment of certain cancers, neurological disorders, depression and potentially improve tumour immunotherapy. The benzo-γ-pyrone scaffold is well-known in medicinal chemistry with diverse pharmacological activities attributed to them, however, their therapeutic potential as adenosine receptor antagonists have not been investigated in detail. To expand on the structure–activity relationships, the present study explored the adenosine A1 and A2A receptor binding affinities of a selected series of benzo-γ-pyrone analogues. In vitro evaluation led to the identification of 5-hydroxy-2-(3-hydroxyphenyl)-4H-1-benzopyran-4-one with the best adenosine A2A receptor affinity among the test compounds and was found to be non-selective (A1Ki = 0.956 µM; A2AKi = 1.44 µM). Hydroxy substitution on ring A and/or B play a key role in modulating the binding affinity at adenosine A1 and A2A receptors. Adenosine A1 receptor affinity was increased to the nanomolar range with hydroxy substitution on C6 (ring A), while meta-hydroxy substitution on ring B governed adenosine A2A receptor affinity. The double bond between C2 and C3 of ring C as well as C2 phenyl substitution was shown to be imperative for both adenosine A1 and A2A receptor affinity. Selected benzo-γ-pyrone derivatives behaved as adenosine A1 receptor antagonists in the performed GTP shift assays. It may be concluded that benzo-γ-pyrone based derivatives are suitable leads for designing and identifying adenosine receptor antagonists as treatment of various disorders.
中文翻译:

苯并吡喃酮是鉴定新型腺苷A 1 / A 2A受体拮抗剂的特权支架
腺苷受体拮抗剂正在作为潜在的候选药物进行研究,以治疗某些癌症,神经系统疾病,抑郁症并可能改善肿瘤免疫疗法。苯并-γ-吡喃酮支架在药物化学中是众所周知的,归因于它们,它们具有多种药理活性,但是,尚未详细研究其作为腺苷受体拮抗剂的治疗潜力。为了扩展结构与活性之间的关系,本研究探索了一系列选定的苯并-γ-吡喃酮类似物的腺苷A 1和A 2A受体结合亲和力。体外评估导致鉴定出具有最佳腺苷A 2A的5-羟基-2-(3-羟基苯基)-4H-1-苯并吡喃-4-酮被测化合物之间的受体亲和力是非选择性的(A 1 K i = 0.956 µM; A 2A K i = 1.44 µM)。环A和/或B上的羟基取代在调节腺苷A 1和A 2A受体的结合亲和力中起关键作用。腺苷A 1受体的亲和力随C6(环A)上的羟基取代而增加到纳摩尔范围,而环B上的间羟基取代决定了腺苷A 2A受体的亲和力。C环C2和C3之间的双键以及C2苯基取代对于腺苷A 1和A 2A都是必不可少的受体亲和力。在执行的GTP位移分析中,选定的苯并-γ-吡喃酮衍生物充当腺苷A 1受体拮抗剂。可以得出结论,基于苯并-γ-吡喃酮的衍生物是设计和鉴定腺苷受体拮抗剂以治疗各种疾病的合适先导。









































 京公网安备 11010802027423号
京公网安备 11010802027423号