Journal of Power Sources ( IF 9.2 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.jpowsour.2017.12.070 Youn Jeong Jang , Jaehyuk Lee , Ju Hun Kim , Byeong Jun Lee , Jae Sung Lee
|
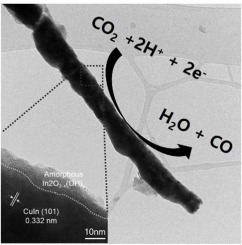
Electrical anodization of Cu foil produces one-dimensional Cu nanowires of high surface areas, which turns to CuIn alloy nanowires by indium electrodeposition replacing edge site Cu atoms. An electrochemical pre-activation forms a highly conformal amorphous In(OH)3 overlayer with oxygen vacancy on the CuIn alloy that facilitates CO2 adsorption to promote selective CO formation suppressing competing H2 adsorption. Thus the activated CuIn alloy nanowires catalyse electrochemical CO2 conversion to CO with high CO selectivity (>68.2%) and high current density (ca. −3.9 mAcm−2) at −0.6 VRHE, which represents the higher partial CO current density (ca. −2.66 mAcm−2) than that of previously reported CuIn alloy powders without nanostructuring. The performance remains stable for more than 15 h without significant degradation.
中文翻译:

一维CuIn合金纳米线作为用于选择性CO 2到CO转化的强大而有效的电催化剂
Cu箔的电阳极氧化产生高表面积的一维Cu纳米线,通过铟电沉积替代边缘位点Cu原子,从而转变为CuIn合金纳米线。电化学预活化在CuIn合金上形成具有氧空位的高度共形的非晶In(OH)3覆盖层,该层有助于CO 2吸附,促进选择性CO的形成,抑制竞争性H 2吸附。因此,活化的CuIn合金纳米线在-0.6 V RHE时以高CO选择性(> 68.2%)和高电流密度(约-3.9 mAcm -2)催化电化学CO 2转化为CO ,这表示较高的部分CO电流密度(约。-2.66 mAcm -2),而不是以前报道的没有纳米结构的CuIn合金粉末。性能可保持稳定超过15小时,而不会出现明显的退化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号