当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redesign of a Grignard-Based Active Pharmaceutical Ingredient (API) Batch Synthesis to a Flow Process for the Preparation of Melitracen HCl
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1021/acs.oprd.7b00368 Michael J. Pedersen 1, 2 , Tommy Skovby 1 , Michael J. Mealy 1 , Kim Dam-Johansen 2 , Søren Kiil 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-26 00:00:00 , DOI: 10.1021/acs.oprd.7b00368 Michael J. Pedersen 1, 2 , Tommy Skovby 1 , Michael J. Mealy 1 , Kim Dam-Johansen 2 , Søren Kiil 2
Affiliation
|
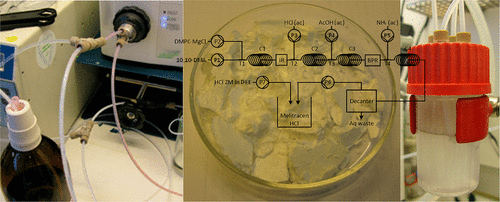
A Grignard-based batch process, for the preparation of Melitracen HCl, has been redesigned to fit a continuous reactor system. The Grignard addition is carried out at room temperature, with subsequent hydrolysis of the magnesium alkoxide intermediate followed by dehydration of the resulting alcohol. The product undergoes further workup by simple gravimetric phase separation and then crystallization with 2 M HCl in diethyl ether to afford pure Melitracen HCl. All steps in the laboratory setup were concatenated, and the setup was proven capable of producing a significant portion of the commercial quantities of Melitracen HCl. The flow setup profits from a reduced footprint, lower energy consumption, fewer synthetic steps, and reduced raw material usage compared to the batch process.
中文翻译:

基于格氏的活性药物成分(API)批次合成的重新设计,以制备盐酸Melitracen HCl的流程
基于格利雅(Grignard)的间歇法,用于制备盐酸Melitracen,已经过重新设计,以适合连续反应器系统。格氏试剂的添加在室温下进行,随后将烷氧基镁中间体水解,然后将所得醇脱水。通过简单的重量相分离对产物进行进一步的处理,然后用2M HCl的乙醚溶液结晶,得到纯的Melitracen HCl。实验室设置中的所有步骤都已连接在一起,并且该设置已被证明能够产生大量商业量的盐酸Melitracen HCl。与批处理流程相比,该流程设置的优点是减少了占地面积,降低了能耗,减少了合成步骤并减少了原材料使用量。
更新日期:2018-01-26
中文翻译:

基于格氏的活性药物成分(API)批次合成的重新设计,以制备盐酸Melitracen HCl的流程
基于格利雅(Grignard)的间歇法,用于制备盐酸Melitracen,已经过重新设计,以适合连续反应器系统。格氏试剂的添加在室温下进行,随后将烷氧基镁中间体水解,然后将所得醇脱水。通过简单的重量相分离对产物进行进一步的处理,然后用2M HCl的乙醚溶液结晶,得到纯的Melitracen HCl。实验室设置中的所有步骤都已连接在一起,并且该设置已被证明能够产生大量商业量的盐酸Melitracen HCl。与批处理流程相比,该流程设置的优点是减少了占地面积,降低了能耗,减少了合成步骤并减少了原材料使用量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号